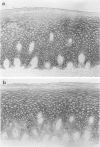
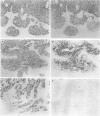
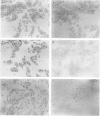
Abstract
Desmosomes are intercellular junctions that have been shown to be down-regulated in certain types of carcinomas and that may play a role in suppression of invasion and metastasis. We have shown previously that immunohistochemical staining for the major desmosomal glycoprotein, desmoglein (Dsg), is reduced in some cases of squamous cell carcinoma (SCC) of the head and neck, and that reduced staining correlates with lymph node involvement. Desmosomes are multicomponent organelles. We therefore sought to determine whether another major desmosomal molecule, desmoplakin (Dp), showed similar reduced expression to that shown by desmoglein. We have stained 65 specimens of primary SCC of the oral cavity (37 non-metastatic and 28 metatastic) with monoclonal antibodies to both desmoglein and desmoplakin. We show that reduction of Dp staining correlates with loss of differentiation of the primary tumour, degree of invasion and presence of lymph node metastases. Similar correlations were found with Dsg staining. There was also correlation between reduction in Dp staining and reduction in Dsg staining. It is concluded that down-regulation of desmosomal expression occurs in some cases of SCC of the oral cavity and is associated with invasion and metastasis. Desmosomes may have an invasion and metastasis suppressor function.
Full text
PDF
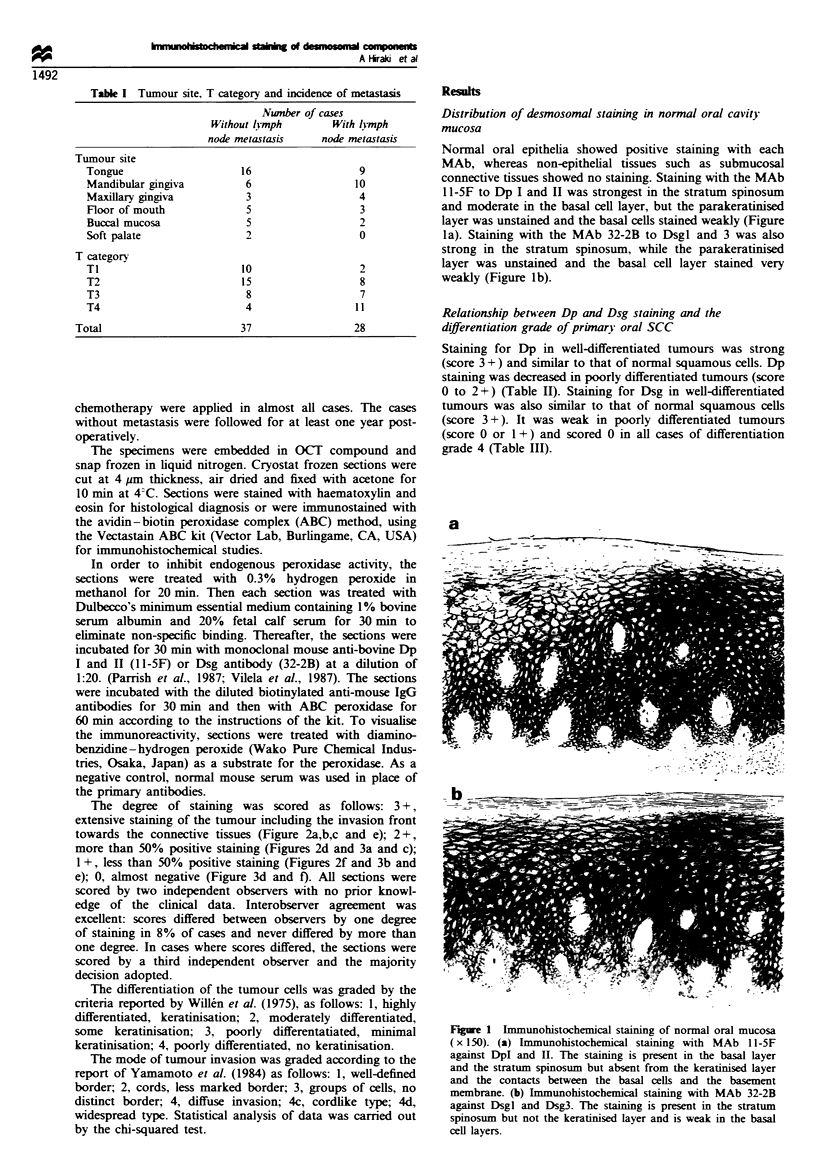

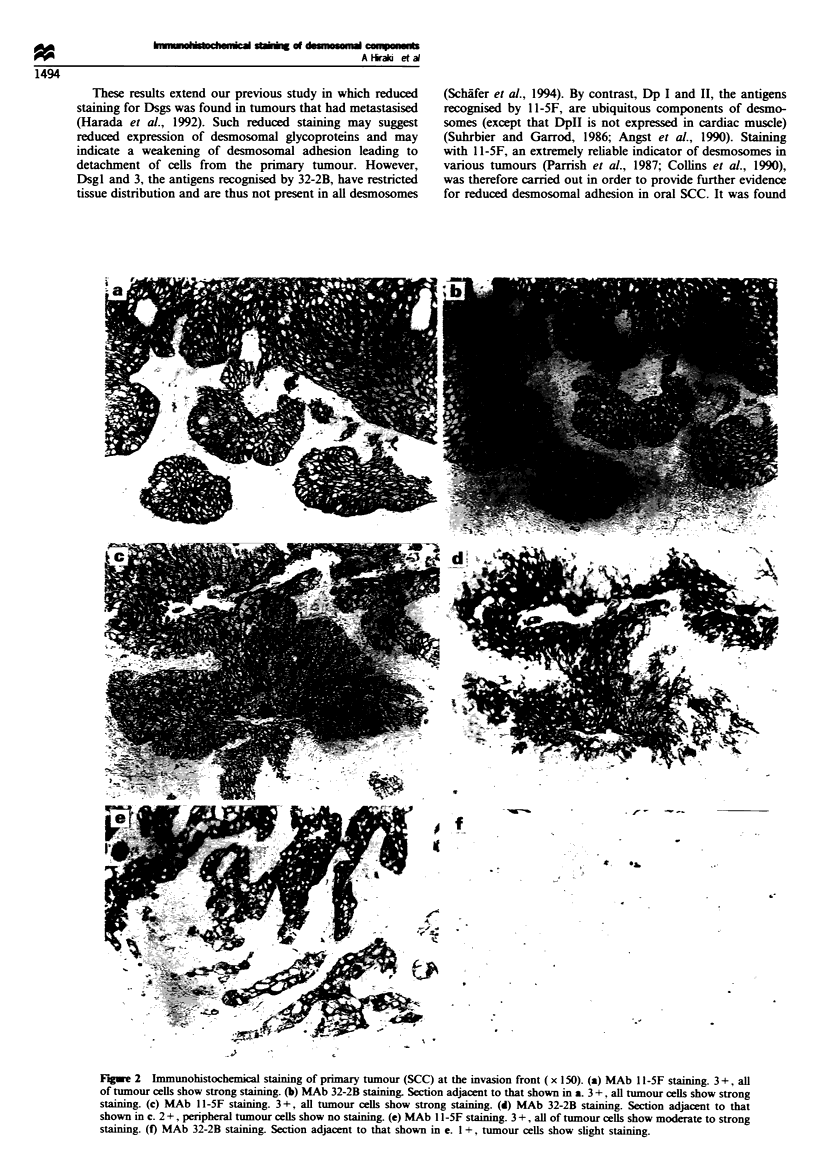
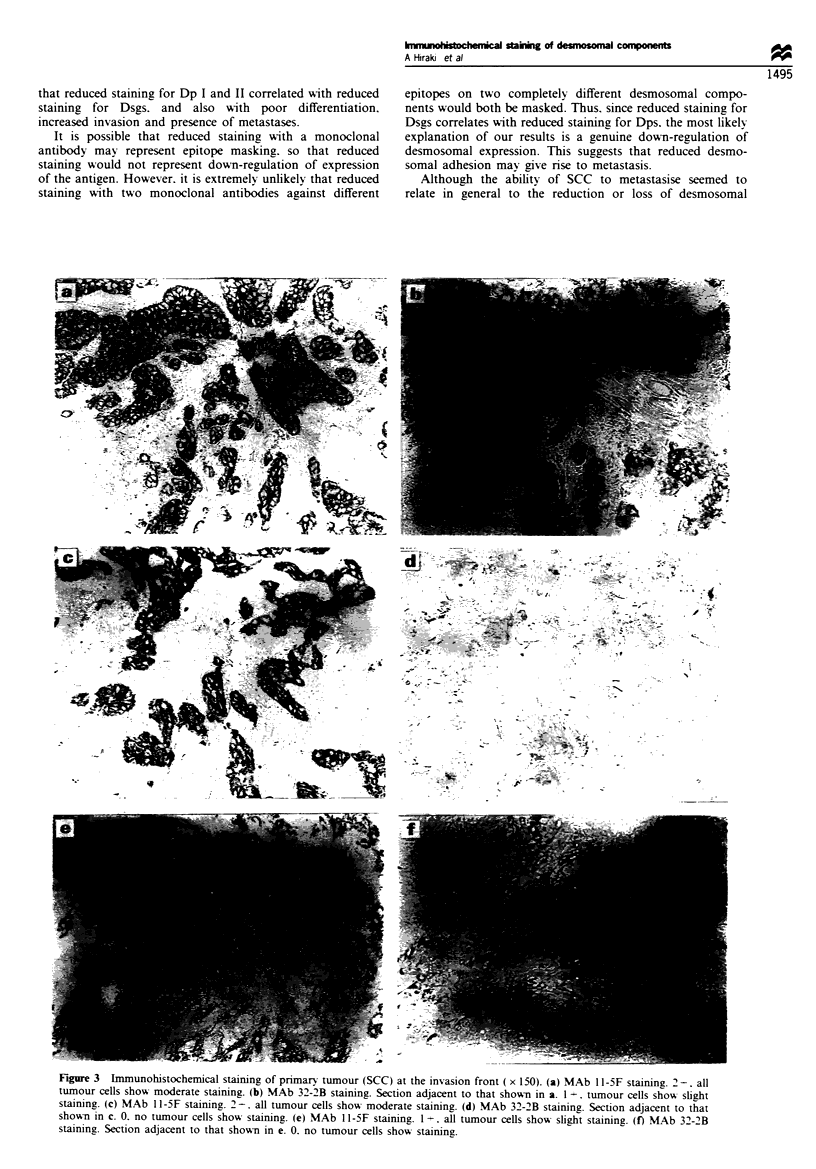


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alroy J., Pauli B. U., Weinstein R. S. Correlation between numbers of desmosomes and the aggressiveness of transitional cell carcinoma in human urinary bladder. Cancer. 1981 Jan 1;47(1):104–112. doi: 10.1002/1097-0142(19810101)47:1<104::aid-cncr2820470118>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Angst B. D., Nilles L. A., Green K. J. Desmoplakin II expression is not restricted to stratified epithelia. J Cell Sci. 1990 Oct;97(Pt 2):247–257. doi: 10.1242/jcs.97.2.247. [DOI] [PubMed] [Google Scholar]
- Birchmeier W., Hülsken J., Behrens J. Adherens junction proteins in tumour progression. Cancer Surv. 1995;24:129–140. [PubMed] [Google Scholar]
- Burkhardt A. Advanced methods in the evaluation of premalignant lesions and carcinomas of the oral mucosa. J Oral Pathol. 1985 Nov;14(10):751–778. doi: 10.1111/j.1600-0714.1985.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Buxton R. S., Cowin P., Franke W. W., Garrod D. R., Green K. J., King I. A., Koch P. J., Magee A. I., Rees D. A., Stanley J. R. Nomenclature of the desmosomal cadherins. J Cell Biol. 1993 May;121(3):481–483. doi: 10.1083/jcb.121.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J. E., Taylor I., Garrod D. R. A study of desmosomes in colorectal carcinoma. Br J Cancer. 1990 Nov;62(5):796–805. doi: 10.1038/bjc.1990.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn I. G., Vilela M. J., Garrod D. R., Crocker J., Wallace D. M. Immunohistochemical staining with monoclonal antibody 32-2B to desmosomal glycoprotein 1. Its role in the histological assessment of urothelial carcinomas. Br J Urol. 1990 Feb;65(2):176–180. doi: 10.1111/j.1464-410x.1990.tb14694.x. [DOI] [PubMed] [Google Scholar]
- Garrod D. R. Desmosomes and cancer. Cancer Surv. 1995;24:97–111. [PubMed] [Google Scholar]
- Garrod D. R. Desmosomes and hemidesmosomes. Curr Opin Cell Biol. 1993 Feb;5(1):30–40. doi: 10.1016/s0955-0674(05)80005-5. [DOI] [PubMed] [Google Scholar]
- Garrod D. R., Parrish E. P., Marston J. E. The structure of desmosomes and their role in malignant disease. Biochem Soc Trans. 1987 Oct;15(5):802–804. doi: 10.1042/bst0150802. [DOI] [PubMed] [Google Scholar]
- Harada T., Shinohara M., Nakamura S., Oka M. An immunohistochemical study of the extracellular matrix in oral squamous cell carcinoma and its association with invasive and metastatic potential. Virchows Arch. 1994;424(3):257–266. doi: 10.1007/BF00194609. [DOI] [PubMed] [Google Scholar]
- Harada T., Shinohara M., Nakamura S., Shimada M., Oka M. Immunohistochemical detection of desmosomes in oral squamous cell carcinomas: correlation with differentiation, mode of invasion, and metastatic potential. Int J Oral Maxillofac Surg. 1992 Dec;21(6):346–349. doi: 10.1016/s0901-5027(05)80759-3. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Amagai M., Watanabe K., Dmochowski M., Chidgey M. A., Yue K. K., Garrod D. R., Nishikawa T. A case of pemphigus vulgaris showing reactivity with pemphigus antigens (Dsg1 and Dsg3) and desmocollins. J Invest Dermatol. 1995 Apr;104(4):541–544. doi: 10.1111/1523-1747.ep12606050. [DOI] [PubMed] [Google Scholar]
- Kouklis P. D., Hutton E., Fuchs E. Making a connection: direct binding between keratin intermediate filaments and desmosomal proteins. J Cell Biol. 1994 Nov;127(4):1049–1060. doi: 10.1083/jcb.127.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk A. P., Stappenbeck T. S., Parry D. A., Palka H. L., Virata M. L., Bornslaeger E. A., Nilles L. A., Green K. J. Structure and function of desmosomal transmembrane core and plaque molecules. Biophys Chem. 1994 May;50(1-2):97–112. doi: 10.1016/0301-4622(94)85023-2. [DOI] [PubMed] [Google Scholar]
- Legan P. K., Yue K. K., Chidgey M. A., Holton J. L., Wilkinson R. W., Garrod D. R. The bovine desmocollin family: a new gene and expression patterns reflecting epithelial cell proliferation and differentiation. J Cell Biol. 1994 Jul;126(2):507–518. doi: 10.1083/jcb.126.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K., Mattey D., Measures H., Hopkins C., Garrod D. Localisation of the protein and glycoprotein components of bovine nasal epithelial desmosomes by immunoelectron microscopy. EMBO J. 1987 Apr;6(4):885–889. doi: 10.1002/j.1460-2075.1987.tb04834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R., Cowin P., Kapprell H. P., Franke W. W. Desmosomal proteins: new markers for identification and classification of tumors. Lab Invest. 1986 Jan;54(1):4–25. [PubMed] [Google Scholar]
- Nuber U. A., Schäfer S., Schmidt A., Koch P. J., Franke W. W. The widespread human desmocollin Dsc2 and tissue-specific patterns of synthesis of various desmocollin subtypes. Eur J Cell Biol. 1995 Jan;66(1):69–74. [PubMed] [Google Scholar]
- O'Keefe E. J., Erickson H. P., Bennett V. Desmoplakin I and desmoplakin II. Purification and characterization. J Biol Chem. 1989 May 15;264(14):8310–8318. [PubMed] [Google Scholar]
- Osborn M., Weber K. A monoclonal antibody recognizing desmosomes: use in human pathology. J Invest Dermatol. 1985 Oct;85(4):385–388. doi: 10.1111/1523-1747.ep12277018. [DOI] [PubMed] [Google Scholar]
- Parrish E. P., Steart P. V., Garrod D. R., Weller R. O. Antidesmosomal monoclonal antibody in the diagnosis of intracranial tumours. J Pathol. 1987 Nov;153(3):265–273. doi: 10.1002/path.1711530311. [DOI] [PubMed] [Google Scholar]
- Pauli B. U., Cohen S. M., Alroy J., Weinstein R. S. Desmosome ultrastructure and biological behavior of chemical carcinogen-induced urinary bladder carcinomas. Cancer Res. 1978 Oct;38(10):3276–3285. [PubMed] [Google Scholar]
- Schindler A. M., Amaudruz M. A., Kocher O., Riotton G., Gabbiani G. Desmosomes and gap-junctions in various epidermoid preneoplastic and neoplastic lesions of the cervix uteri. Acta Cytol. 1982 Jul-Aug;26(4):466–470. [PubMed] [Google Scholar]
- Schipper J. H., Frixen U. H., Behrens J., Unger A., Jahnke K., Birchmeier W. E-cadherin expression in squamous cell carcinomas of head and neck: inverse correlation with tumor dedifferentiation and lymph node metastasis. Cancer Res. 1991 Dec 1;51(23 Pt 1):6328–6337. [PubMed] [Google Scholar]
- Schmidt A., Heid H. W., Schäfer S., Nuber U. A., Zimbelmann R., Franke W. W. Desmosomes and cytoskeletal architecture in epithelial differentiation: cell type-specific plaque components and intermediate filament anchorage. Eur J Cell Biol. 1994 Dec;65(2):229–245. [PubMed] [Google Scholar]
- Schäfer S., Koch P. J., Franke W. W. Identification of the ubiquitous human desmoglein, Dsg2, and the expression catalogue of the desmoglein subfamily of desmosomal cadherins. Exp Cell Res. 1994 Apr;211(2):391–399. doi: 10.1006/excr.1994.1103. [DOI] [PubMed] [Google Scholar]
- Suhrbier A., Garrod D. An investigation of the molecular components of desmosomes in epithelial cells of five vertebrates. J Cell Sci. 1986 Mar;81:223–242. doi: 10.1242/jcs.81.1.223. [DOI] [PubMed] [Google Scholar]
- Vilela M. J., Parrish E. P., Wright D. H., Garrod D. R. Monoclonal antibody to desmosomal glycoprotein 1--a new epithelial marker for diagnostic pathology. J Pathol. 1987 Dec;153(4):365–375. doi: 10.1002/path.1711530410. [DOI] [PubMed] [Google Scholar]
- Willén R., Nathanson A., Moberger G., Anneroth G. Squamous cell carcinoma of the gingiva. Histological classification and grading of malignancy. Acta Otolaryngol. 1975 Jan-Feb;79(1-2):146–154. doi: 10.3109/00016487509124667. [DOI] [PubMed] [Google Scholar]
- Yamamoto E., Miyakawa A., Kohama G. Mode of invasion and lymph node metastasis in squamous cell carcinoma of the oral cavity. Head Neck Surg. 1984 May-Jun;6(5):938–947. doi: 10.1002/hed.2890060508. [DOI] [PubMed] [Google Scholar]
- Yue K. K., Holton J. L., Clarke J. P., Hyam J. L., Hashimoto T., Chidgey M. A., Garrod D. R. Characterisation of a desmocollin isoform (bovine DSC3) exclusively expressed in lower layers of stratified epithelia. J Cell Sci. 1995 Jun;108(Pt 6):2163–2173. doi: 10.1242/jcs.108.6.2163. [DOI] [PubMed] [Google Scholar]