Abstract
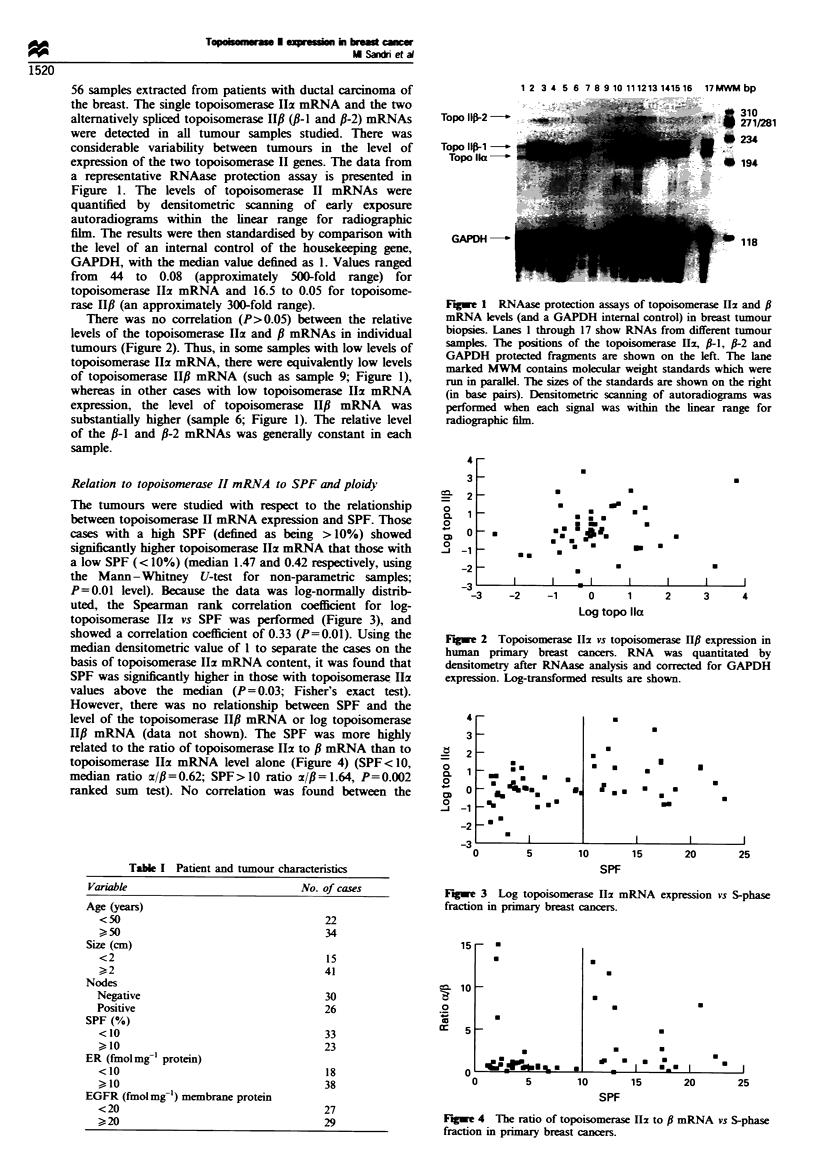
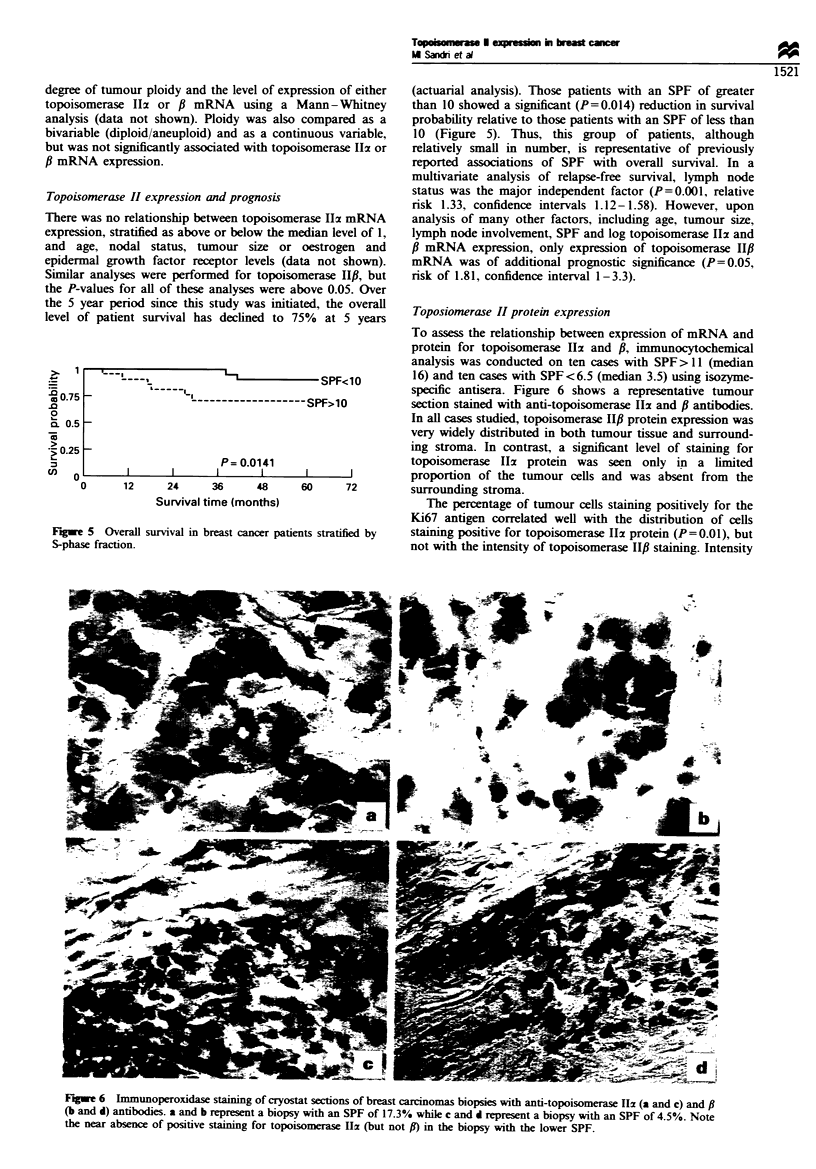
Topoisomerase II is a key target for several anti-cancer drugs used for breast cancer therapy, including doxorubicin, epirubicin and mitoxantrone. Two isoforms of topoisomerase II (alpha and beta) have been described in human cells which differ in their subcellular localisation, biochemical properties and susceptibility to inhibition by anti-cancer drugs. The relative level of expression of the alpha and beta isoforms may contribute to the degree of tumour responsiveness to different chemotherapeutic agents. To assess the relationship between expression of topoisomerase II isoforms and established prognostic factors and pathological variables, 56 primary breast tumour samples were studied. The expression of the two topoisomerase II genes was apparently not co-ordinately regulated in these tissue samples. There was no relationship between any of the commonly used pathological variables [tumour size, lymph node status, S-phase fraction (SPF)] and the level of expression of topoisomerase II beta mRNA. However, high topoisomerase II alpha gene expression was significantly associated with a high SPF (sign-rank test; P = 0.01). Moreover, the ratio of mRNA levels for topoisomerase II alpha and beta showed a stronger relationship to SPF (median raito 0.62 for tumours with SPF < 10, and 1.64 for SPF > 10; P = 0.0021, sign-rank test). As expected from previous studies, an SPF > 10 was associated with poor overall survival (P = 0.01). Immunohistochemical analysis revealed that topoisomerase II beta was widely distributed ( > 90% positive tumour cells), but that topoisomerase II alpha expression was less widely expressed, with a pattern of expression similar to that of the proliferation-dependent antigen recognised by Ki67. Because topoisomerase II gene expression showed a log-normal distribution, log-transformed data were used in multivariate analysis of relapse-free survival. This showed that lymph node status and topoisomerase II beta mRNA expression were the only significant survival factors (P = 0.001 and 0.05, respectively, with relative risks of 1.3 and 1.8). These results indicate that topoisomerase II alpha, but not beta, expression is dependent upon cellular proliferation status, but that the more widely expressed topoisomerase II beta protein may play a significant role as a target for anti-tumour therapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. C., Clark G. M., Elledge R., Fuqua S. A., Brown R. W., Chamness G. C., Osborne C. K., McGuire W. L. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993 Feb 3;85(3):200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- Austin C. A., Sng J. H., Patel S., Fisher L. M. Novel HeLa topoisomerase II is the II beta isoform: complete coding sequence and homology with other type II topoisomerases. Biochim Biophys Acta. 1993 Mar 20;1172(3):283–291. doi: 10.1016/0167-4781(93)90215-y. [DOI] [PubMed] [Google Scholar]
- Beck W. T., Danks M. K., Wolverton J. S., Kim R., Chen M. Drug resistance associated with altered DNA topoisomerase II. Adv Enzyme Regul. 1993;33:113–127. doi: 10.1016/0065-2571(93)90012-3. [DOI] [PubMed] [Google Scholar]
- Camplejohn R. S., Macartney J. C., Morris R. W. Measurement of S-phase fractions in lymphoid tissue comparing fresh versus paraffin-embedded tissue and 4',6'-diamidino-2 phenylindole dihydrochloride versus propidium iodide staining. Cytometry. 1989 Jul;10(4):410–416. doi: 10.1002/cyto.990100408. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung T. D., Drake F. H., Tan K. B., Per S. R., Crooke S. T., Mirabelli C. K. Characterization and immunological identification of cDNA clones encoding two human DNA topoisomerase II isozymes. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9431–9435. doi: 10.1073/pnas.86.23.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou A., Henning-Chubb C., Huberman E. Novobiocin- and phorbol-12-myristate-13-acetate-induced differentiation of human leukemia cells associated with a reduction in topoisomerase II activity. Cancer Res. 1989 Mar 1;49(5):1110–1117. [PubMed] [Google Scholar]
- Davies S. L., Jenkins J. R., Hickson I. D. Human cells express two differentially spliced forms of topoisomerase II beta mRNA. Nucleic Acids Res. 1993 Aug 11;21(16):3719–3723. doi: 10.1093/nar/21.16.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. M., Robson C. N., Davies S. L., Hickson I. D. Nuclear topoisomerase II levels correlate with the sensitivity of mammalian cells to intercalating agents and epipodophyllotoxins. J Biol Chem. 1988 Nov 25;263(33):17724–17729. [PubMed] [Google Scholar]
- Drake F. H., Hofmann G. A., Bartus H. F., Mattern M. R., Crooke S. T., Mirabelli C. K. Biochemical and pharmacological properties of p170 and p180 forms of topoisomerase II. Biochemistry. 1989 Oct 3;28(20):8154–8160. doi: 10.1021/bi00446a029. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Halligan B., Cooke C. A., Heck M. M., Liu L. F. Topoisomerase II is a structural component of mitotic chromosome scaffolds. J Cell Biol. 1985 May;100(5):1706–1715. doi: 10.1083/jcb.100.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B., Gunduz N., Costantino J., Fisher E. R., Redmond C., Mamounas E. P., Siderits R. DNA flow cytometric analysis of primary operable breast cancer. Relation of ploidy and S-phase fraction to outcome of patients in NSABP B-04. Cancer. 1991 Oct 1;68(7):1465–1475. doi: 10.1002/1097-0142(19911001)68:7<1465::aid-cncr2820680702>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Fry A. M., Chresta C. M., Davies S. M., Walker M. C., Harris A. L., Hartley J. A., Masters J. R., Hickson I. D. Relationship between topoisomerase II level and chemosensitivity in human tumor cell lines. Cancer Res. 1991 Dec 15;51(24):6592–6595. [PubMed] [Google Scholar]
- Gasparini G., Pozza F., Harris A. L. Evaluating the potential usefulness of new prognostic and predictive indicators in node-negative breast cancer patients. J Natl Cancer Inst. 1993 Aug 4;85(15):1206–1219. doi: 10.1093/jnci/85.15.1206. [DOI] [PubMed] [Google Scholar]
- Gekeler V., Frese G., Noller A., Handgretinger R., Wilisch A., Schmidt H., Muller C. P., Dopfer R., Klingebiel T., Diddens H. Mdr1/P-glycoprotein, topoisomerase, and glutathione-S-transferase pi gene expression in primary and relapsed state adult and childhood leukaemias. Br J Cancer. 1992 Sep;66(3):507–517. doi: 10.1038/bjc.1992.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Gerdes J., Li L., Schlueter C., Duchrow M., Wohlenberg C., Gerlach C., Stahmer I., Kloth S., Brandt E., Flad H. D. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991 Apr;138(4):867–873. [PMC free article] [PubMed] [Google Scholar]
- Harris A. L., Nicholson S., Sainsbury J. R., Farndon J., Wright C. Epidermal growth factor receptors in breast cancer: association with early relapse and death, poor response to hormones and interactions with neu. J Steroid Biochem. 1989;34(1-6):123–131. doi: 10.1016/0022-4731(89)90072-1. [DOI] [PubMed] [Google Scholar]
- Hellemans P., van Dam P. A., Geyskens M., van Oosterom A. T., Buytaert P., Van Marck E. Immunohistochemical study of topoisomerase II-alpha expression in primary ductal carcinoma of the breast. J Clin Pathol. 1995 Feb;48(2):147–150. doi: 10.1136/jcp.48.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiang Y. H., Wu H. Y., Liu L. F. Proliferation-dependent regulation of DNA topoisomerase II in cultured human cells. Cancer Res. 1988 Jun 1;48(11):3230–3235. [PubMed] [Google Scholar]
- Ishikawa H., Kawano M. M., Okada K., Tanaka H., Tanabe O., Sakai A., Asaoku H., Iwato K., Nobuyoshi M., Kuramoto A. Expressions of DNA topoisomerase I and II gene and the genes possibly related to drug resistance in human myeloma cells. Br J Haematol. 1993 Jan;83(1):68–74. doi: 10.1111/j.1365-2141.1993.tb04633.x. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R., Ayton P., Jones T., Davies S. L., Simmons D. L., Harris A. L., Sheer D., Hickson I. D. Isolation of cDNA clones encoding the beta isozyme of human DNA topoisomerase II and localisation of the gene to chromosome 3p24. Nucleic Acids Res. 1992 Nov 11;20(21):5587–5592. doi: 10.1093/nar/20.21.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Karp J. E., Jones R. J., Miller C. B., Schneider E., Zwelling L. A., Cowan K., Wendel K., Burke P. J. Topoisomerase II levels and drug sensitivity in adult acute myelogenous leukemia. Blood. 1994 Jan 15;83(2):517–530. [PubMed] [Google Scholar]
- Keith W. N., Douglas F., Wishart G. C., McCallum H. M., George W. D., Kaye S. B., Brown R. Co-amplification of erbB2, topoisomerase II alpha and retinoic acid receptor alpha genes in breast cancer and allelic loss at topoisomerase I on chromosome 20. Eur J Cancer. 1993;29A(10):1469–1475. doi: 10.1016/0959-8049(93)90022-8. [DOI] [PubMed] [Google Scholar]
- Kim R., Hirabayashi N., Nishiyama M., Yorishima T., Toge T., Okada K. mRNA expression of topoisomerase II in human tumors and normal tissues. Jpn J Surg. 1991 Sep;21(5):587–589. doi: 10.1007/BF02471001. [DOI] [PubMed] [Google Scholar]
- McGuire W. L., Clark G. M. Prognostic factors and treatment decisions in axillary-node-negative breast cancer. N Engl J Med. 1992 Jun 25;326(26):1756–1761. doi: 10.1056/NEJM199206253262607. [DOI] [PubMed] [Google Scholar]
- McLeod H. L., Douglas F., Oates M., Symonds R. P., Prakash D., van der Zee A. G., Kaye S. B., Brown R., Keith W. N. Topoisomerase I and II activity in human breast, cervix, lung and colon cancer. Int J Cancer. 1994 Dec 1;59(5):607–611. doi: 10.1002/ijc.2910590506. [DOI] [PubMed] [Google Scholar]
- O'Reilly S. M., Camplejohn R. S., Barnes D. M., Millis R. R., Rubens R. D., Richards M. A. Node-negative breast cancer: prognostic subgroups defined by tumor size and flow cytometry. J Clin Oncol. 1990 Dec;8(12):2040–2046. doi: 10.1200/JCO.1990.8.12.2040. [DOI] [PubMed] [Google Scholar]
- O'Reilly S. M., Richards M. A. Is DNA flow cytometry a useful investigation in breast cancer? Eur J Cancer. 1992;28(2-3):504–507. doi: 10.1016/s0959-8049(05)80088-7. [DOI] [PubMed] [Google Scholar]
- Osheroff N., Zechiedrich E. L., Gale K. C. Catalytic function of DNA topoisomerase II. Bioessays. 1991 Jun;13(6):269–273. doi: 10.1002/bies.950130603. [DOI] [PubMed] [Google Scholar]
- Potmesil M., Hsiang Y. H., Liu L. F., Bank B., Grossberg H., Kirschenbaum S., Forlenza T. J., Penziner A., Kanganis D., Forlenzar T. J. Resistance of human leukemic and normal lymphocytes to drug-induced DNA cleavage and low levels of DNA topoisomerase II. Cancer Res. 1988 Jun 15;48(12):3537–3543. [PubMed] [Google Scholar]
- Prosperi E., Negri C., Marchese G., Ricotti G. C. Expression of the 170-kDa and 180-kDa isoforms of DNA topoisomerase II in resting and proliferating human lymphocytes. Cell Prolif. 1994 May;27(5):257–267. doi: 10.1111/j.1365-2184.1994.tb01423.x. [DOI] [PubMed] [Google Scholar]
- Roberge M., Gasser S. M. DNA loops: structural and functional properties of scaffold-attached regions. Mol Microbiol. 1992 Feb;6(4):419–423. doi: 10.1111/j.1365-2958.1992.tb01485.x. [DOI] [PubMed] [Google Scholar]
- Smith K., Houlbrook S., Greenall M., Carmichael J., Harris A. L. Topoisomerase II alpha co-amplification with erbB2 in human primary breast cancer and breast cancer cell lines: relationship to m-AMSA and mitoxantrone sensitivity. Oncogene. 1993 Apr;8(4):933–938. [PubMed] [Google Scholar]
- Smith P. J., Makinson T. A. Cellular consequences of overproduction of DNA topoisomerase II in an ataxia-telangiectasia cell line. Cancer Res. 1989 Mar 1;49(5):1118–1124. [PubMed] [Google Scholar]
- Verheijen R., Kuijpers H. J., van Driel R., Beck J. L., van Dierendonck J. H., Brakenhoff G. J., Ramaekers F. C. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. II. Localization in mitotic cells and association with chromosomes. J Cell Sci. 1989 Apr;92(Pt 4):531–540. doi: 10.1242/jcs.92.4.531. [DOI] [PubMed] [Google Scholar]
- Watt P. M., Hickson I. D. Structure and function of type II DNA topoisomerases. Biochem J. 1994 Nov 1;303(Pt 3):681–695. doi: 10.1042/bj3030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C. D., Latham M. D., Lock R. B., Sullivan D. M. Attenuated topoisomerase II content directly correlates with a low level of drug resistance in a Chinese hamster ovary cell line. Cancer Res. 1991 Dec 15;51(24):6543–6549. [PubMed] [Google Scholar]
- Wells N. J., Addison C. M., Fry A. M., Ganapathi R., Hickson I. D. Serine 1524 is a major site of phosphorylation on human topoisomerase II alpha protein in vivo and is a substrate for casein kinase II in vitro. J Biol Chem. 1994 Nov 25;269(47):29746–29751. [PubMed] [Google Scholar]
- Woessner R. D., Chung T. D., Hofmann G. A., Mattern M. R., Mirabelli C. K., Drake F. H., Johnson R. K. Differences between normal and ras-transformed NIH-3T3 cells in expression of the 170kD and 180kD forms of topoisomerase II. Cancer Res. 1990 May 15;50(10):2901–2908. [PubMed] [Google Scholar]
- Woessner R. D., Mattern M. R., Mirabelli C. K., Johnson R. K., Drake F. H. Proliferation- and cell cycle-dependent differences in expression of the 170 kilodalton and 180 kilodalton forms of topoisomerase II in NIH-3T3 cells. Cell Growth Differ. 1991 Apr;2(4):209–214. [PubMed] [Google Scholar]
- Zwelling L. A., Hinds M., Chan D., Altschuler E., Mayes J., Zipf T. F. Phorbol ester effects on topoisomerase II activity and gene expression in HL-60 human leukemia cells with different proclivities toward monocytoid differentiation. Cancer Res. 1990 Nov 15;50(22):7116–7122. [PubMed] [Google Scholar]