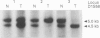Abstract
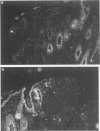
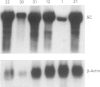
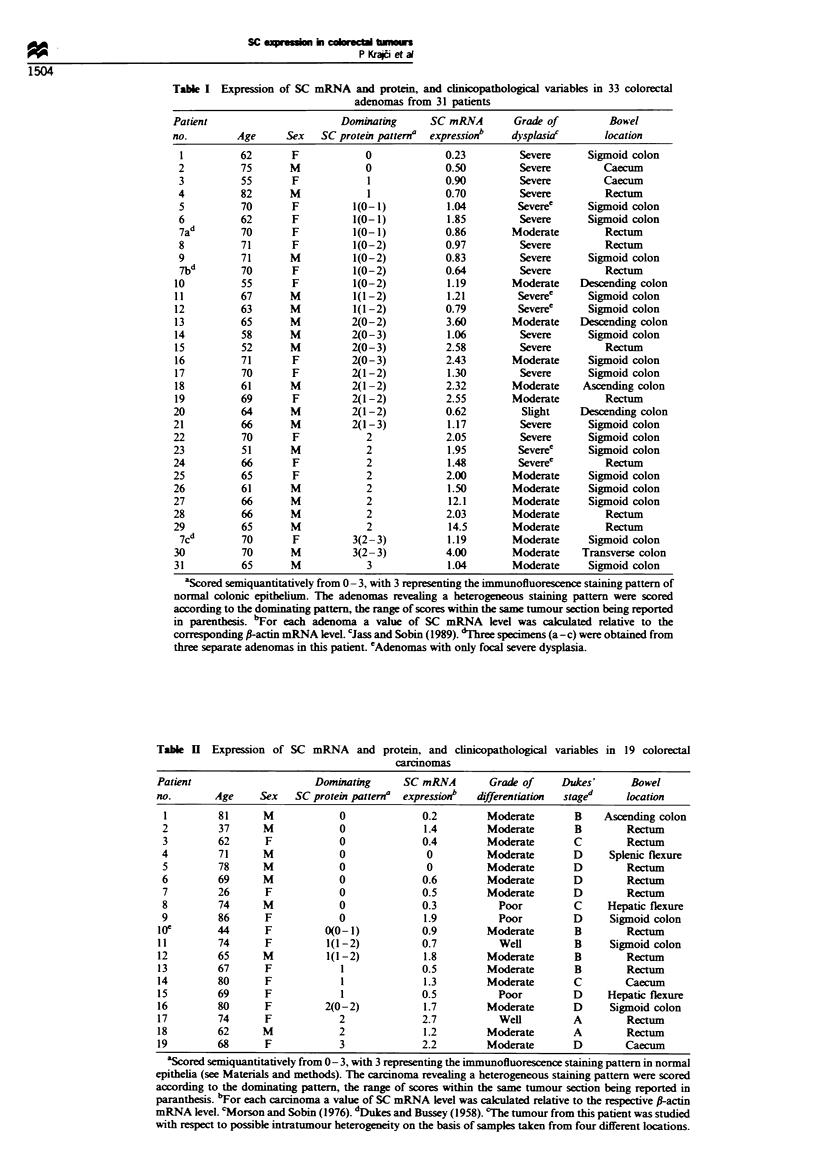
Secretary component (SC) is expressed basolaterally as a transmembrane protein (pIg receptor) on secretory epithelial cells. As pIg receptor it plays a central role in humoral immunity by mediating the external translocation of dimeric IgA and pentameric IgM. A few case reports have suggested that reduced or absent SC protein expression is associated with diarrhoeal disease, but there is no convincing evidence that a primary pIg receptor deficiency can occur. In this study the relative presence of SC mRNA was determined by Northern blot analysis and related to immunohistochemically determined SC protein expression in 33 colorectal adenomas (31 patients) with increased risk of developing sporadic colorectal cancer, as well as in 19 colorectal carcinomas from 19 patients with such sporadic tumours. In the adenomas, SC mRNA levels were positively related to SC protein expression; both mRNA and SC protein were negatively related to histological grade. Similarly, SC mRNA levels tended to be related to the SC protein expression in the carcinomas. SC mRNA was detected in all adenomas, and only two of ten carcinomas (10.5%) deemed to be SC deficient by immunohistochemistry also lacked SC mRNA expression, suggesting diallelic alterations in the SC-encoding gene (locus PIGR). This possibility agreed with Southern blot analysis performed on a separate sample of 32 other colonic carcinomas in which the diallelic loss of D1S58 (which exhibits a close linkage centromerically to PIGR) was calculated to be 6.4%. Together these findings suggested that reduced SC protein expression in colorectal adenomas might be a transcriptional defect reflecting the degree of cellular dysplasia, whereas absent SC protein expression in colorectal carcinomas might also involve post-transcriptional defects and occasional diallelic gene deletions representing late events in carcinogenesis.
Full text
PDF
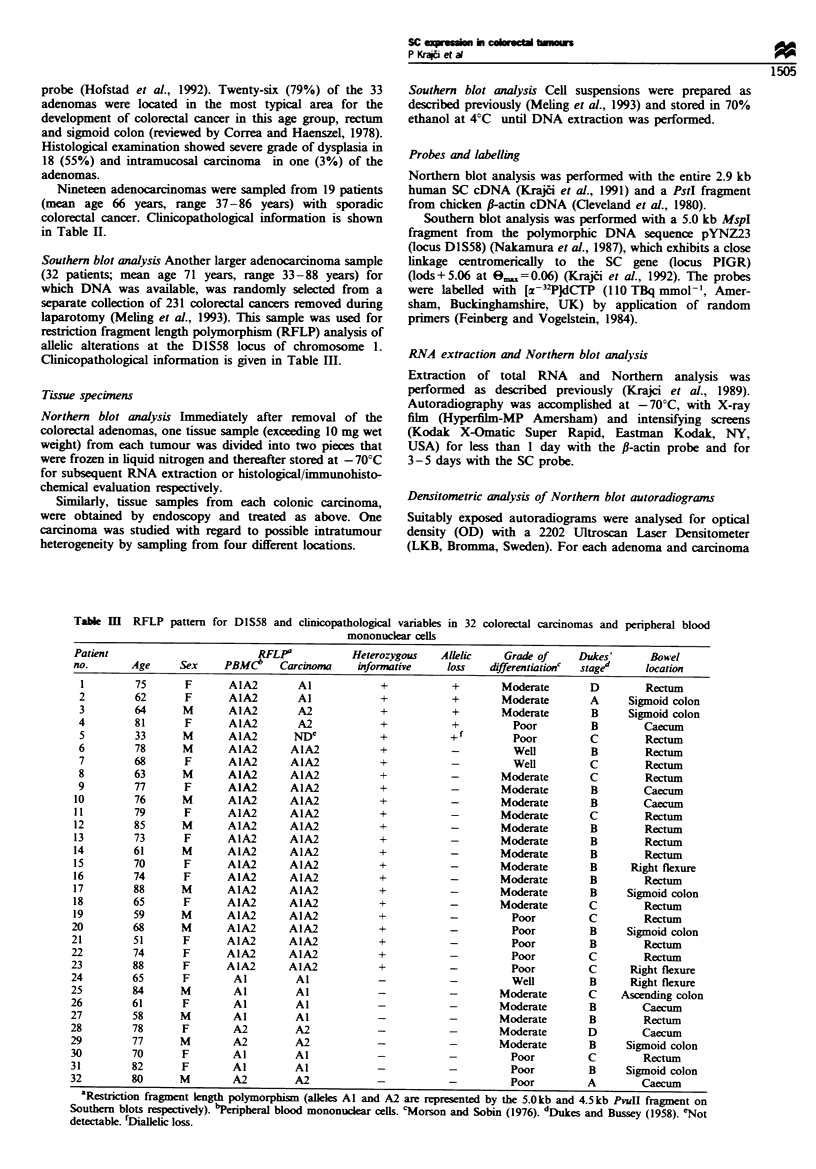
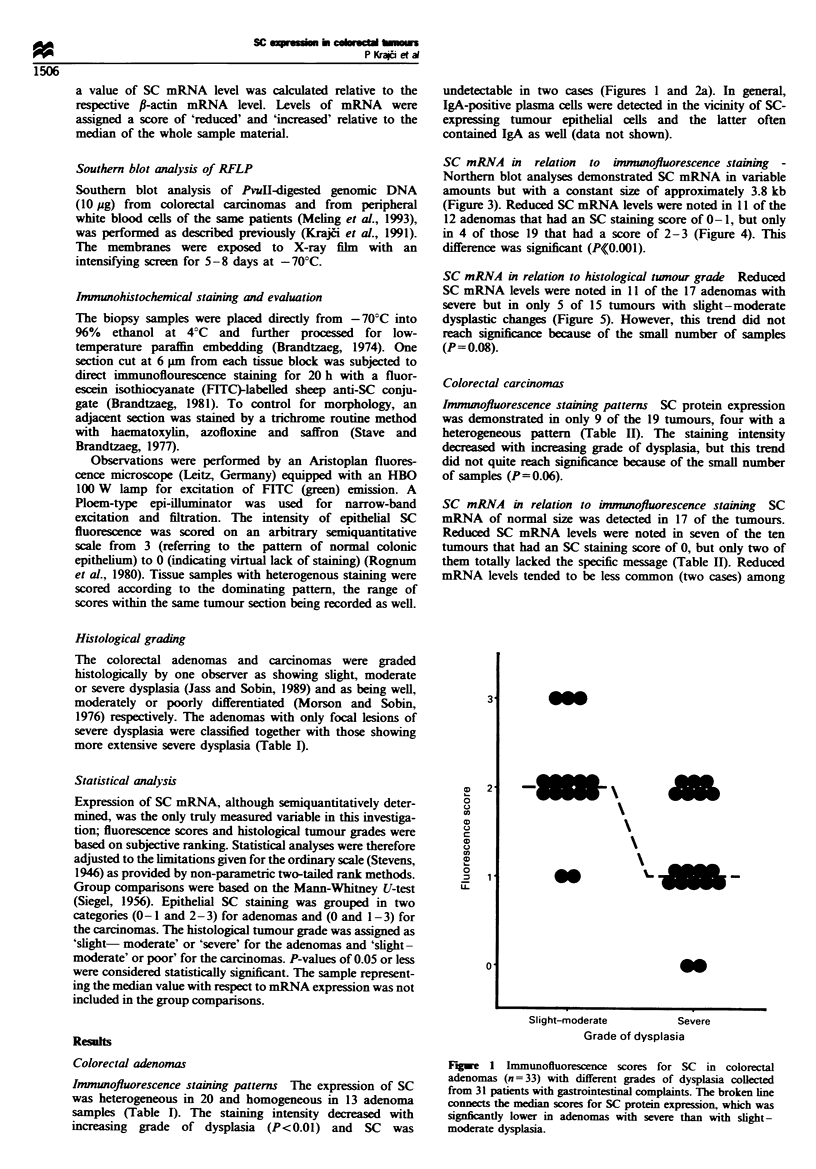
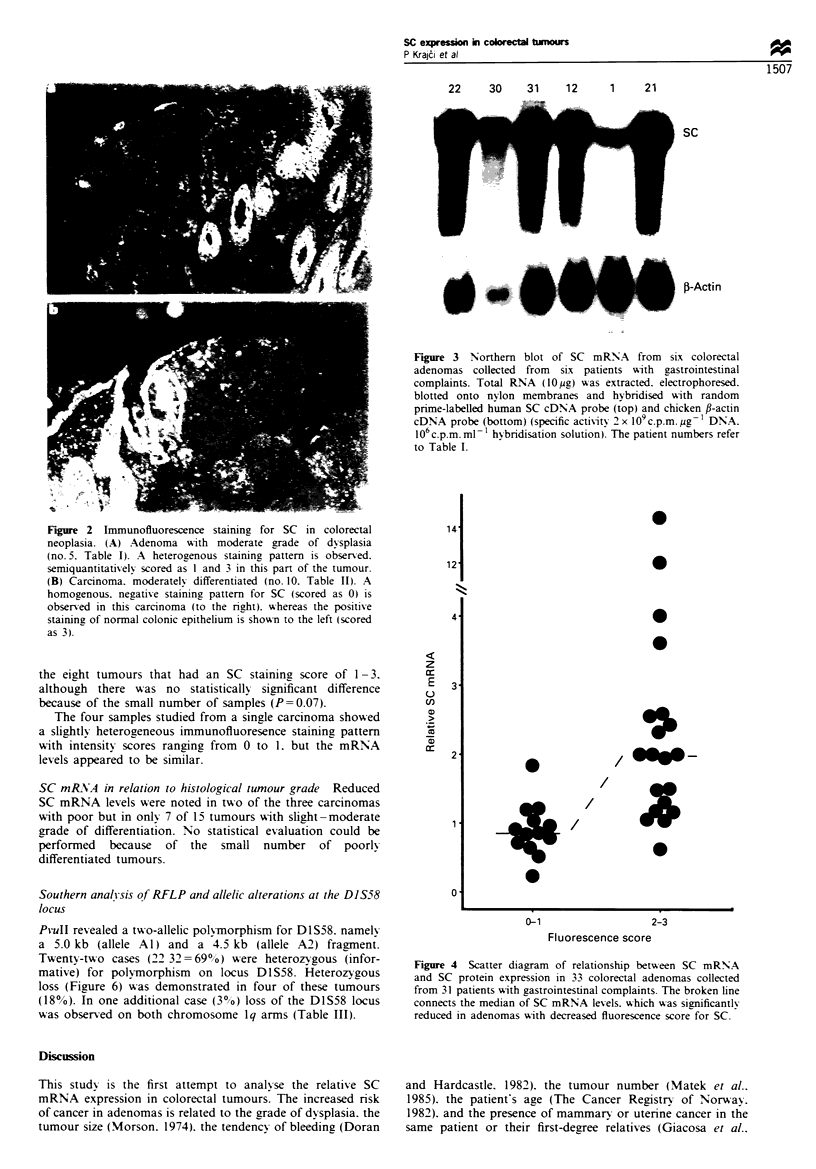
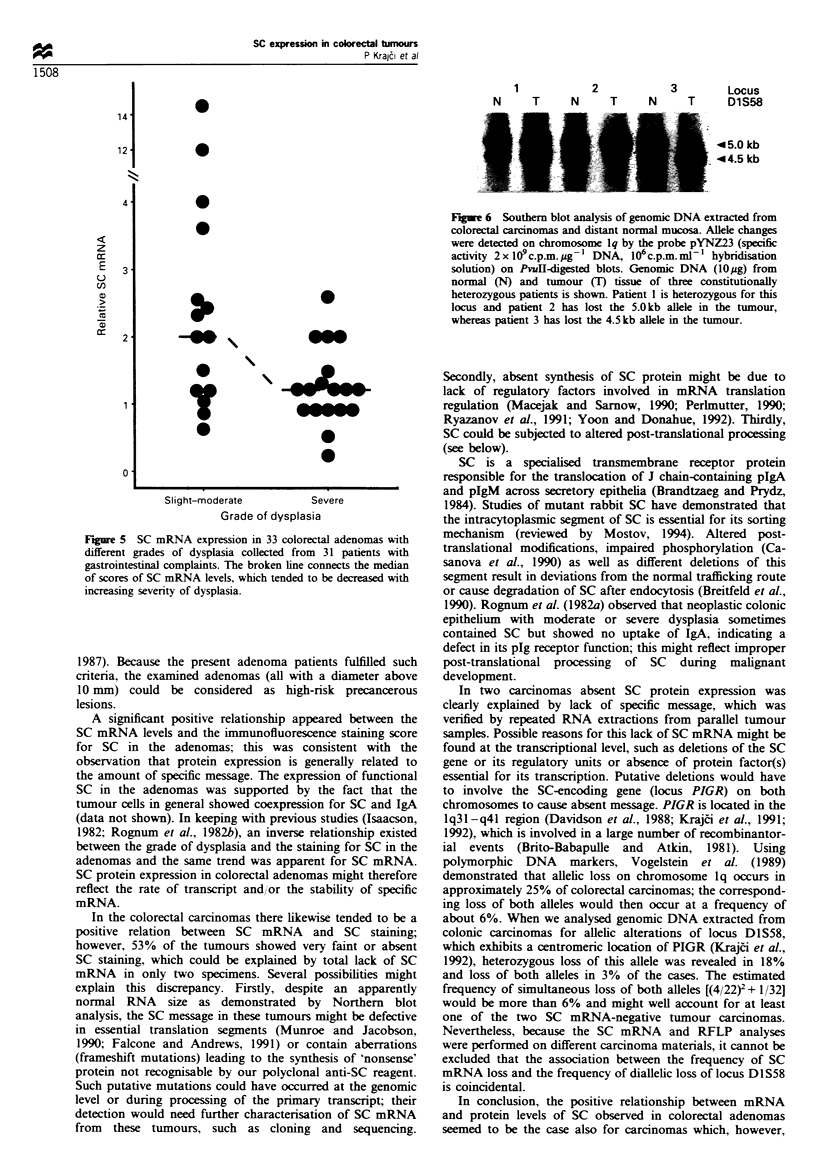


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components. Immunohistochemistry with a cold ethanol-fixation technique. Immunology. 1974 Jun;26(6):1101–1114. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Nilssen D. E., Rognum T. O., Thrane P. S. Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol Clin North Am. 1991 Sep;20(3):397–439. [PubMed] [Google Scholar]
- Brandtzaeg P., Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature. 1984 Sep 6;311(5981):71–73. doi: 10.1038/311071a0. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Role of J chain and secretory component in receptor-mediated glandular and hepatic transport of immunoglobulins in man. Scand J Immunol. 1985 Aug;22(2):111–146. doi: 10.1111/j.1365-3083.1985.tb01866.x. [DOI] [PubMed] [Google Scholar]
- Breitfeld P. P., Casanova J. E., McKinnon W. C., Mostov K. E. Deletions in the cytoplasmic domain of the polymeric immunoglobulin receptor differentially affect endocytotic rate and postendocytotic traffic. J Biol Chem. 1990 Aug 15;265(23):13750–13757. [PubMed] [Google Scholar]
- Brito-Babapulle V., Atkin N. B. Break points in chromosome #1 abnormalities of 218 human neoplasms. Cancer Genet Cytogenet. 1981 Nov;4(3):215–225. doi: 10.1016/0165-4608(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Casanova J. E., Breitfeld P. P., Ross S. A., Mostov K. E. Phosphorylation of the polymeric immunoglobulin receptor required for its efficient transcytosis. Science. 1990 May 11;248(4956):742–745. doi: 10.1126/science.2110383. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Conley M. E., Delacroix D. L. Intravascular and mucosal immunoglobulin A: two separate but related systems of immune defense? Ann Intern Med. 1987 Jun;106(6):892–899. doi: 10.7326/0003-4819-106-6-892. [DOI] [PubMed] [Google Scholar]
- Correa P., Haenszel W. The epidemiology of large-bowel cancer. Adv Cancer Res. 1978;26:1–141. doi: 10.1016/s0065-230x(08)60086-x. [DOI] [PubMed] [Google Scholar]
- DUKES C. E., BUSSEY H. J. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958 Sep;12(3):309–320. doi: 10.1038/bjc.1958.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. K., Le Beau M. M., Eddy R. L., Shows T. B., DiPietro L. A., Kingzette M., Hanly W. C. Genetic mapping of the human polymeric immunoglobulin receptor gene to chromosome region 1q31----q41. Cytogenet Cell Genet. 1988;48(2):107–111. doi: 10.1159/000132601. [DOI] [PubMed] [Google Scholar]
- Doran J., Hardcastle J. D. Bleeding patterns in colorectal cancer: the effect of aspirin and the implications for faecal occult blood testing. Br J Surg. 1982 Dec;69(12):711–713. doi: 10.1002/bjs.1800691209. [DOI] [PubMed] [Google Scholar]
- Falcone D., Andrews D. W. Both the 5' untranslated region and the sequences surrounding the start site contribute to efficient initiation of translation in vitro. Mol Cell Biol. 1991 May;11(5):2656–2664. doi: 10.1128/mcb.11.5.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Hoff G., Moen I. E., Trygg K., Frølich W., Sauar J., Vatn M., Gjone E., Larsen S. Epidemiology of polyps in the rectum and sigmoid colon. Evaluation of nutritional factors. Scand J Gastroenterol. 1986 Mar;21(2):199–204. doi: 10.3109/00365528609034647. [DOI] [PubMed] [Google Scholar]
- Hofstad B., Vatn M., Larsen S., Osnes M. Reliability of in situ measurements of colorectal polyps. Scand J Gastroenterol. 1992;27(1):59–64. doi: 10.3109/00365529209011168. [DOI] [PubMed] [Google Scholar]
- Isaacson P. Immunoperoxidase study of the secretory immunoglobulin system in colonic neoplasia. J Clin Pathol. 1982 Jan;35(1):14–25. doi: 10.1136/jcp.35.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretz K., Schlag P., Quentmeier A., Möller P. Evaluation of the secretory component as a prognostic variable in colorectal carcinoma. Int J Cancer. 1994 May 1;57(3):365–370. doi: 10.1002/ijc.2910570313. [DOI] [PubMed] [Google Scholar]
- Krajci P., Gedde-Dahl T., Jr, Høyheim B., Rogde S., Olaisen B., Brandtzaeg P. The gene encoding human transmembrane secretory component (locus PIGR) is linked to D1S58 on chromosome 1. Hum Genet. 1992 Nov;90(3):215–219. doi: 10.1007/BF00220065. [DOI] [PubMed] [Google Scholar]
- Krajci P., Grzeschik K. H., Geurts van Kessel A. H., Olaisen B., Brandtzaeg P. The human transmembrane secretory component (poly-Ig receptor): molecular cloning, restriction fragment length polymorphism and chromosomal sublocalization. Hum Genet. 1991 Oct;87(6):642–648. doi: 10.1007/BF00201717. [DOI] [PubMed] [Google Scholar]
- Krajci P., Solberg R., Sandberg M., Oyen O., Jahnsen T., Brandtzaeg P. Molecular cloning of the human transmembrane secretory component (poly-Ig receptor) and its mRNA expression in human tissues. Biochem Biophys Res Commun. 1989 Feb 15;158(3):783–789. doi: 10.1016/0006-291x(89)92790-3. [DOI] [PubMed] [Google Scholar]
- Krajci P., Taskén K., Kvale D., Brandtzaeg P. Interferon-gamma stimulation of messenger RNA for human secretory component (poly-Ig receptor) depends on continuous intermediate protein synthesis. Scand J Immunol. 1993 Feb;37(2):251–256. doi: 10.1111/j.1365-3083.1993.tb01763.x. [DOI] [PubMed] [Google Scholar]
- Krakauer R., Zinneman H. H., Hong R. Deficiency of secretory Ig-A and intestinal malabsorption. Am J Gastroenterol. 1975 Oct;64(4):319–323. [PubMed] [Google Scholar]
- Kvale D., Brandtzaeg P., Løvhaug D. Up-regulation of the expression of secretory component and HLA molecules in a human colonic cell line by tumour necrosis factor-alpha and gamma interferon. Scand J Immunol. 1988 Sep;28(3):351–357. doi: 10.1111/j.1365-3083.1988.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Macejak D. G., Sarnow P. Translational regulation of the immunoglobulin heavy-chain binding protein mRNA. Enzyme. 1990;44(1-4):310–319. doi: 10.1159/000468767. [DOI] [PubMed] [Google Scholar]
- Matek W., Guggenmoos-Holzmann I., Demling L. Follow-up of patients with colorectal adenomas. Endoscopy. 1985 Sep;17(5):175–181. doi: 10.1055/s-2007-1018494. [DOI] [PubMed] [Google Scholar]
- Meling G. I., Lothe R. A., Børresen A. L., Graue C., Hauge S., Clausen O. P., Rognum T. O. The TP53 tumour suppressor gene in colorectal carcinomas. I. Genetic alterations on chromosome 17. Br J Cancer. 1993 Jan;67(1):88–92. doi: 10.1038/bjc.1993.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morson B. President's address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974 Jun;67(6 Pt 1):451–457. doi: 10.1177/00359157740676P115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., Blobel G. A transmembrane precursor of secretory component. The receptor for transcellular transport of polymeric immunoglobulins. J Biol Chem. 1982 Oct 10;257(19):11816–11821. [PubMed] [Google Scholar]
- Mostov K. E. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- Munroe D., Jacobson A. mRNA poly(A) tail, a 3' enhancer of translational initiation. Mol Cell Biol. 1990 Jul;10(7):3441–3455. doi: 10.1128/mcb.10.7.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Culver M., Gillilan S., O'Connell P., Leppert M., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence pYNZ23 to chromosome 1 (DIS58). Nucleic Acids Res. 1987 Nov 25;15(22):9620–9620. doi: 10.1093/nar/15.22.9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinson E., Lahav M., Berebi A., Estrov Z., Zur S., Resnitzky P. Secretory piece and IgA deficiency in a patient with Waldenstrom's macroglobulinemia. Am J Gastroenterol. 1986 Oct;81(10):995–998. [PubMed] [Google Scholar]
- Perlmutter R. M. Translational regulation of the lymphocyte-specific protein tyrosine kinase p56lck. Enzyme. 1990;44(1-4):214–224. doi: 10.1159/000468759. [DOI] [PubMed] [Google Scholar]
- Phillips J. O., Everson M. P., Moldoveanu Z., Lue C., Mestecky J. Synergistic effect of IL-4 and IFN-gamma on the expression of polymeric Ig receptor (secretory component) and IgA binding by human epithelial cells. J Immunol. 1990 Sep 15;145(6):1740–1744. [PubMed] [Google Scholar]
- Piskurich J. F., France J. A., Tamer C. M., Willmer C. A., Kaetzel C. S., Kaetzel D. M. Interferon-gamma induces polymeric immunoglobulin receptor mRNA in human intestinal epithelial cells by a protein synthesis dependent mechanism. Mol Immunol. 1993 Mar;30(4):413–421. doi: 10.1016/0161-5890(93)90071-i. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Ridker P. New light on secretory-component deficiency. N Engl J Med. 1992 Jul 9;327(2):129–129. doi: 10.1056/nejm199207093270216. [DOI] [PubMed] [Google Scholar]
- Rognum T. O., Elgjo K., Fausa O., Brandtzaeg P. Immunohistochemical evaluation of carcinoembryonic antigen, secretory component, and epithelial IgA in ulcerative colitis with dysplasia. Gut. 1982 Feb;23(2):123–133. doi: 10.1136/gut.23.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryazanov A. G., Rudkin B. B., Spirin A. S. Regulation of protein synthesis at the elongation stage. New insights into the control of gene expression in eukaryotes. FEBS Lett. 1991 Jul 22;285(2):170–175. doi: 10.1016/0014-5793(91)80798-8. [DOI] [PubMed] [Google Scholar]
- Scott H., Brandtzaeg P., Solheim B. G., Thorsby E. Relation between HLA-DR-like antigens and secretory component (SC) in jejunal epithelium of patients with coeliac disease or dermatitis herpetiformis. Clin Exp Immunol. 1981 May;44(2):233–238. [PMC free article] [PubMed] [Google Scholar]
- Sollid L. M., Gaudernack G., Markussen G., Kvale D., Brandtzaeg P., Thorsby E. Induction of various HLA class II molecules in a human colonic adenocarcinoma cell line. Scand J Immunol. 1987 Feb;25(2):175–180. doi: 10.1111/j.1365-3083.1987.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Stave R., Brandtzaeg P. Fluorescence staining of gastric mucosa. A study with special reference to parietal cells. Scand J Gastroenterol. 1977;12(7):885–891. doi: 10.3109/00365527709181735. [DOI] [PubMed] [Google Scholar]
- Stevens S. S. On the Theory of Scales of Measurement. Science. 1946 Jun 7;103(2684):677–680. doi: 10.1126/science.103.2684.677. [DOI] [PubMed] [Google Scholar]
- Strober W., Krakauer R., Klaeveman H. L., Reynolds H. Y., Nelson D. L. Secretory component deficiency. A disorder of the IgA immune system. N Engl J Med. 1976 Feb 12;294(7):351–356. doi: 10.1056/NEJM197602122940701. [DOI] [PubMed] [Google Scholar]
- Thrane P. S., Sollid L. M., Haanes H. R., Brandtzaeg P. Clustering of IgA-producing immunocytes related to HLA-DR-positive ducts in normal and inflamed salivary glands. Scand J Immunol. 1992 Jan;35(1):43–51. doi: 10.1111/j.1365-3083.1992.tb02832.x. [DOI] [PubMed] [Google Scholar]
- Valnes K., Brandtzaeg P., Elgjo K., Stave R. Specific and nonspecific humoral defense factors in the epithelium of normal and inflamed gastric mucosa. Immunohistochemical localization of immunoglobulins, secretory component, lysozyme, and lactoferrin. Gastroenterology. 1984 Mar;86(3):402–412. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Yoon H., Donahue T. F. Control of translation initiation in Saccharomyces cerevisiae. Mol Microbiol. 1992 Jun;6(11):1413–1419. doi: 10.1111/j.1365-2958.1992.tb00861.x. [DOI] [PubMed] [Google Scholar]