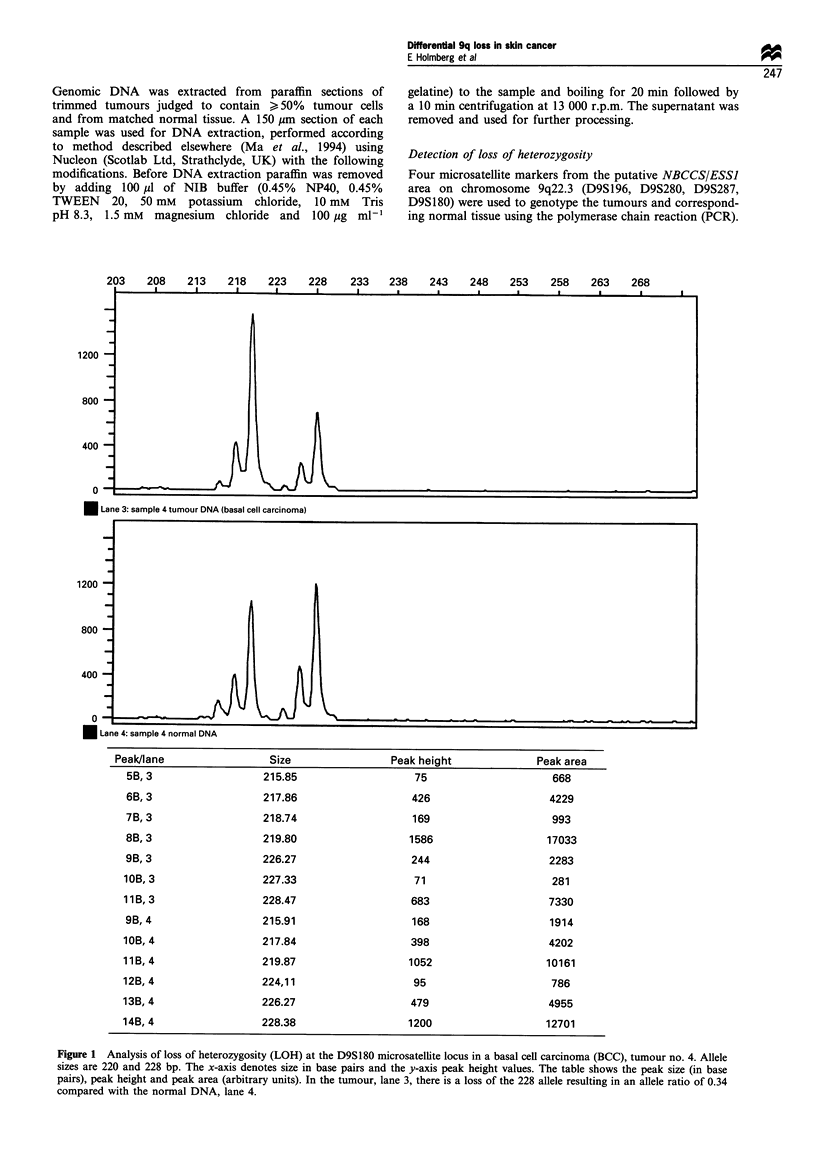
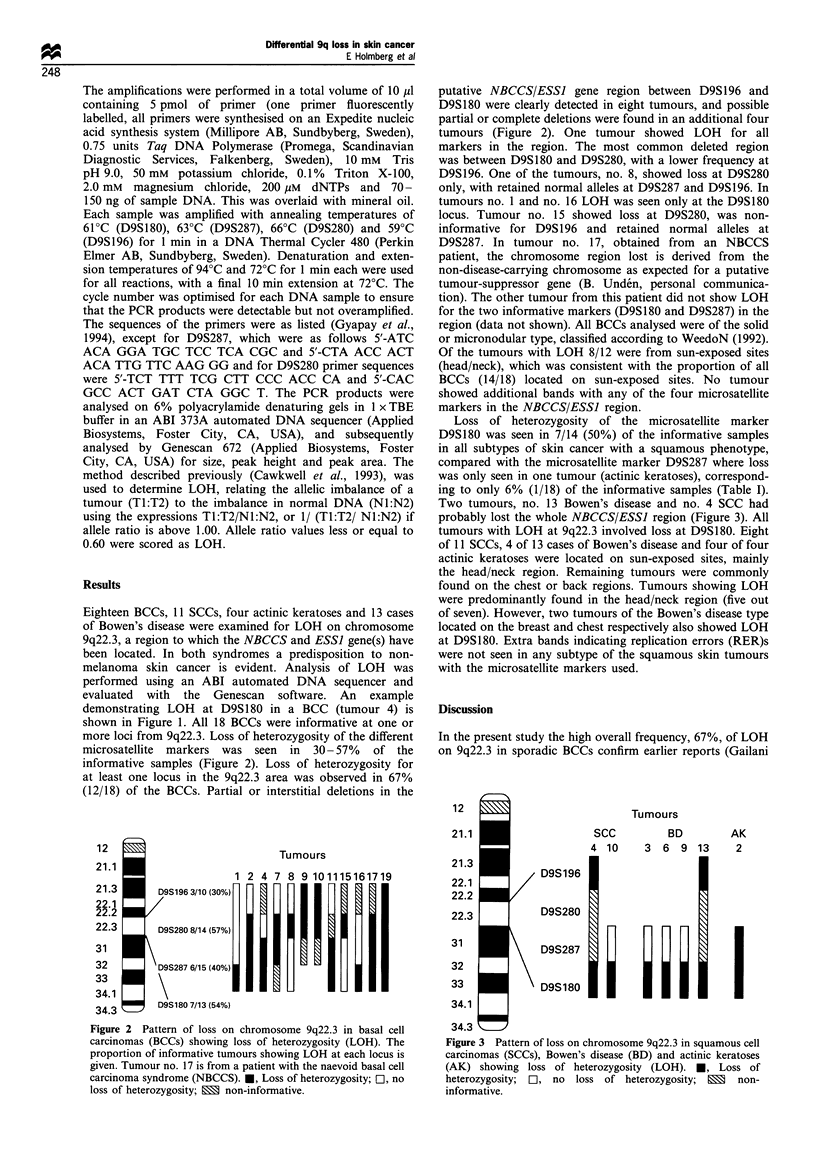
Abstract
Familial predisposition to basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) of the skin are apparent in the autosomal dominant syndromes naevoid basal cell carcinoma syndrome (NBCCS) and multiple self-healing squamous epitheliomata (MSSE) respectively. The gene responsible for NBCCS has been proposed to be a tumour-suppressor gene and is mapped to the same 2 Mb interval on 9q22.3 as the MSSE gene ESS1. In an attempt to further map the NBCCS gene, we have examined loss of heterozygosity (LOH) in 16 sporadic BCCs and two familial BCCs using microsatellite markers located within the candidate gene region. The overall frequency of LOH observed was 67% in the BCCs and partial or interstitial deletions were found in eight tumours, with the highest LOH frequency at markers D9S280, D9S287 and D9S180. To determine if the same genomic region also shows frequent LOH in tumours with a squamous phenotype, we have examined 11 SCCs, four actinic keratoses and 13 cases of Bowen's disease for LOH at 9q22.3. An overall LOH frequency of 50% was observed at D9S180, and occurred in all types of squamous tumours. In contrast, a much lower LOH frequency of only 6% was found at the D9S287 locus. Our observation of different patterns of LOH at 9q22.3 in sporadic BCCs and SCCs implies that more than one tumour-suppressor gene might be located in this genomic region.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ah-See K. W., Cooke T. G., Pickford I. R., Soutar D., Balmain A. An allelotype of squamous carcinoma of the head and neck using microsatellite markers. Cancer Res. 1994 Apr 1;54(7):1617–1621. [PubMed] [Google Scholar]
- Bonifas J. M., Bare J. W., Kerschmann R. L., Master S. P., Epstein E. H., Jr Parental origin of chromosome 9q22.3-q31 lost in basal cell carcinomas from basal cell nevus syndrome patients. Hum Mol Genet. 1994 Mar;3(3):447–448. doi: 10.1093/hmg/3.3.447. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Rudolph J. A., Simon J. A., Lin A., McKenna G. J., Baden H. P., Halperin A. J., Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawkwell L., Bell S. M., Lewis F. A., Dixon M. F., Taylor G. R., Quirke P. Rapid detection of allele loss in colorectal tumours using microsatellites and fluorescent DNA technology. Br J Cancer. 1993 Jun;67(6):1262–1267. doi: 10.1038/bjc.1993.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenevix-Trench G., Wicking C., Berkman J., Sharpe H., Hockey A., Haan E., Oley C., Ravine D., Turner A., Goldgar D. Further localization of the gene for nevoid basal cell carcinoma syndrome (NBCCS) in 15 Australasian families: linkage and loss of heterozygosity. Am J Hum Genet. 1993 Sep;53(3):760–767. [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Ladusans E. J., Rimmer S., Burnell L. D., Thakker N., Farndon P. A. Complications of the naevoid basal cell carcinoma syndrome: results of a population based study. J Med Genet. 1993 Jun;30(6):460–464. doi: 10.1136/jmg.30.6.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndon P. A., Del Mastro R. G., Evans D. G., Kilpatrick M. W. Location of gene for Gorlin syndrome. Lancet. 1992 Mar 7;339(8793):581–582. doi: 10.1016/0140-6736(92)90868-4. [DOI] [PubMed] [Google Scholar]
- Farndon P. A., Morris D. J., Hardy C., McConville C. M., Weissenbach J., Kilpatrick M. W., Reis A. Analysis of 133 meioses places the genes for nevoid basal cell carcinoma (Gorlin) syndrome and Fanconi anemia group C in a 2.6-cM interval and contributes to the fine map of 9q22.3. Genomics. 1994 Sep 15;23(2):486–489. doi: 10.1006/geno.1994.1528. [DOI] [PubMed] [Google Scholar]
- Gailani M. R., Bale S. J., Leffell D. J., DiGiovanna J. J., Peck G. L., Poliak S., Drum M. A., Pastakia B., McBride O. W., Kase R. Developmental defects in Gorlin syndrome related to a putative tumor suppressor gene on chromosome 9. Cell. 1992 Apr 3;69(1):111–117. doi: 10.1016/0092-8674(92)90122-s. [DOI] [PubMed] [Google Scholar]
- Gallagher R. P., Hill G. B., Bajdik C. D., Coldman A. J., Fincham S., McLean D. I., Threlfall W. J. Sunlight exposure, pigmentation factors, and risk of nonmelanocytic skin cancer. II. Squamous cell carcinoma. Arch Dermatol. 1995 Feb;131(2):164–169. [PubMed] [Google Scholar]
- Gallagher R. P., Hill G. B., Bajdik C. D., Fincham S., Coldman A. J., McLean D. I., Threlfall W. J. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer. I. Basal cell carcinoma. Arch Dermatol. 1995 Feb;131(2):157–163. [PubMed] [Google Scholar]
- Goldstein A. M., Stewart C., Bale A. E., Bale S. J., Dean M. Localization of the gene for the nevoid basal cell carcinoma syndrome. Am J Hum Genet. 1994 May;54(5):765–773. [PMC free article] [PubMed] [Google Scholar]
- Gorlin R. J. Nevoid basal-cell carcinoma syndrome. Medicine (Baltimore) 1987 Mar;66(2):98–113. doi: 10.1097/00005792-198703000-00002. [DOI] [PubMed] [Google Scholar]
- Goudie D. R., Yuille M. A., Leversha M. A., Furlong R. A., Carter N. P., Lush M. J., Affara N. A., Ferguson-Smith M. A. Multiple self-healing squamous epitheliomata (ESS1) mapped to chromosome 9q22-q31 in families with common ancestry. Nat Genet. 1993 Feb;3(2):165–169. doi: 10.1038/ng0293-165. [DOI] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Habuchi T., Devlin J., Elder P. A., Knowles M. A. Detailed deletion mapping of chromosome 9q in bladder cancer: evidence for two tumour suppressor loci. Oncogene. 1995 Oct 19;11(8):1671–1674. [PubMed] [Google Scholar]
- Ko C. B., Walton S., Keczkes K., Bury H. P., Nicholson C. The emerging epidemic of skin cancer. Br J Dermatol. 1994 Mar;130(3):269–272. doi: 10.1111/j.1365-2133.1994.tb02920.x. [DOI] [PubMed] [Google Scholar]
- Konishi K., Yamanishi K., Ishizaki K., Yamada K., Kishimoto S., Yasuno H. Analysis of p53 gene mutations and loss of heterozygosity for loci on chromosome 9q in basal cell carcinoma. Cancer Lett. 1994 Apr 29;79(1):67–72. doi: 10.1016/0304-3835(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Kwa R. E., Campana K., Moy R. L. Biology of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992 Jan;26(1):1–26. doi: 10.1016/0190-9622(92)70001-v. [DOI] [PubMed] [Google Scholar]
- Lieu F. M., Yamanishi K., Konishi K., Kishimoto S., Yasuno H. Low incidence of Ha-ras oncogene mutations in human epidermal tumors. Cancer Lett. 1991 Sep;59(3):231–235. doi: 10.1016/0304-3835(91)90146-9. [DOI] [PubMed] [Google Scholar]
- Ma H. W., Cheng J., Caddy B. Extraction of high quality genomic DNA from microsamples of human blood. J Forensic Sci Soc. 1994 Oct-Dec;34(4):231–235. doi: 10.1016/s0015-7368(94)72925-x. [DOI] [PubMed] [Google Scholar]
- Miller S. J. Biology of basal cell carcinoma (Part I). J Am Acad Dermatol. 1991 Jan;24(1):1–13. doi: 10.1016/0190-9622(91)70001-i. [DOI] [PubMed] [Google Scholar]
- Miura K., Okita K., Furukawa Y., Matsuno S., Nakamura Y. Deletion mapping in squamous cell carcinomas of the esophagus defines a region containing a tumor suppressor gene within a 4-centimorgan interval of the distal long arm of chromosome 9. Cancer Res. 1995 May 1;55(9):1828–1830. [PubMed] [Google Scholar]
- Mori T., Yanagisawa A., Kato Y., Miura K., Nishihira T., Mori S., Nakamura Y. Accumulation of genetic alterations during esophageal carcinogenesis. Hum Mol Genet. 1994 Nov;3(11):1969–1971. doi: 10.1093/hmg/3.11.1969. [DOI] [PubMed] [Google Scholar]
- Povey S., Armour J., Farndon P., Haines J. L., Knowles M., Olopade F., Pilz A., White J. A., Kwiatkowski D. J. Report and abstracts of the Third International Workshop on Chromosome 9. Cambridge, United Kingdom, 9-11 April, 1994. Ann Hum Genet. 1994 Jul;58(Pt 3):177–250. doi: 10.1111/j.1469-1809.1994.tb01887.x. [DOI] [PubMed] [Google Scholar]
- Quinn A. G., Campbell C., Healy E., Rees J. L. Chromosome 9 allele loss occurs in both basal and squamous cell carcinomas of the skin. J Invest Dermatol. 1994 Mar;102(3):300–303. doi: 10.1111/1523-1747.ep12371786. [DOI] [PubMed] [Google Scholar]
- Quinn A. G., Healy E., Rehman I., Sikkink S., Rees J. L. Microsatellite instability in human non-melanoma and melanoma skin cancer. J Invest Dermatol. 1995 Mar;104(3):309–312. doi: 10.1111/1523-1747.ep12664612. [DOI] [PubMed] [Google Scholar]
- Quinn A. G., Sikkink S., Rees J. L. Basal cell carcinomas and squamous cell carcinomas of human skin show distinct patterns of chromosome loss. Cancer Res. 1994 Sep 1;54(17):4756–4759. [PubMed] [Google Scholar]
- Quinn A. G., Sikkink S., Rees J. L. Delineation of two distinct deleted regions on chromosome 9 in human non-melanoma skin cancers. Genes Chromosomes Cancer. 1994 Dec;11(4):222–225. doi: 10.1002/gcc.2870110404. [DOI] [PubMed] [Google Scholar]
- Rehman I., Quinn A. G., Healy E., Rees J. L. High frequency of loss of heterozygosity in actinic keratoses, a usually benign disease. Lancet. 1994 Sep 17;344(8925):788–789. doi: 10.1016/s0140-6736(94)92343-4. [DOI] [PubMed] [Google Scholar]
- Reis A., Küster W., Linss G., Gebel E., Hamm H., Fuhrmann W., Wolff G., Groth W., Gustafson G., Kuklik M. Localisation of gene for the naevoid basal-cell carcinoma syndrome. Lancet. 1992 Mar 7;339(8793):617–617. doi: 10.1016/0140-6736(92)90903-g. [DOI] [PubMed] [Google Scholar]
- Schofield D., West D. C., Anthony D. C., Marshal R., Sklar J. Correlation of loss of heterozygosity at chromosome 9q with histological subtype in medulloblastomas. Am J Pathol. 1995 Feb;146(2):472–480. [PMC free article] [PubMed] [Google Scholar]
- Shanley S. M., Dawkins H., Wainwright B. J., Wicking C., Heenan P., Eldon M., Searle J., Chenevix-Trench G. Fine deletion mapping on the long arm of chromosome 9 in sporadic and familial basal cell carcinomas. Hum Mol Genet. 1995 Jan;4(1):129–133. doi: 10.1093/hmg/4.1.129. [DOI] [PubMed] [Google Scholar]
- Wicking C., Berkman J., Wainwright B., Chenevix-Trench G. Fine genetic mapping of the gene for nevoid basal cell carcinoma syndrome. Genomics. 1994 Aug;22(3):505–511. doi: 10.1006/geno.1994.1423. [DOI] [PubMed] [Google Scholar]
- Zaphiropoulos P. G., Söderkvist P., Hedblad M. A., Toftgård R. Genetic instability of microsatellite markers in region q22.3-q31 of chromosome 9 in skin squamous cell carcinomas. Biochem Biophys Res Commun. 1994 Jun 30;201(3):1495–1501. doi: 10.1006/bbrc.1994.1873. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Leffell D. J., Kunala S., Sharma H. W., Gailani M., Simon J. A., Halperin A. J., Baden H. P., Shapiro P. E., Bale A. E. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Riet P., Karp D., Farmer E., Wei Q., Grossman L., Tokino K., Ruppert J. M., Sidransky D. Progression of basal cell carcinoma through loss of chromosome 9q and inactivation of a single p53 allele. Cancer Res. 1994 Jan 1;54(1):25–27. [PubMed] [Google Scholar]



