Abstract
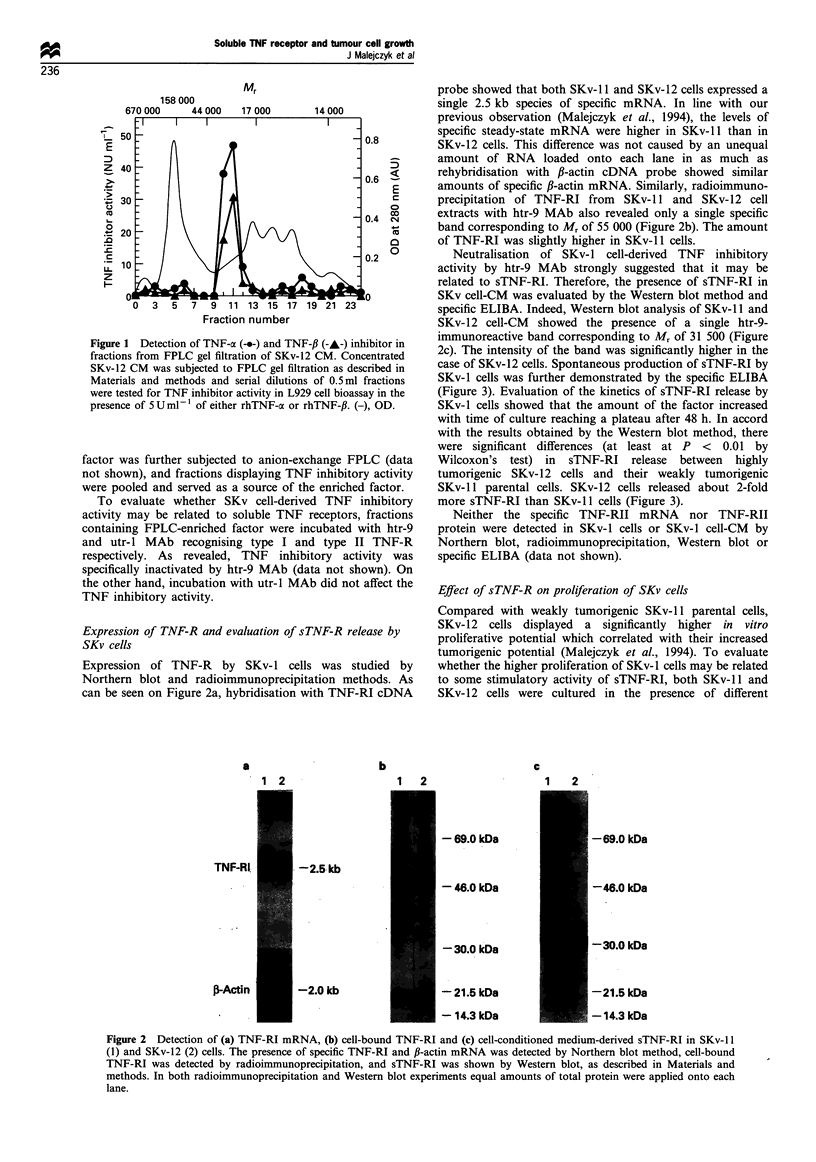
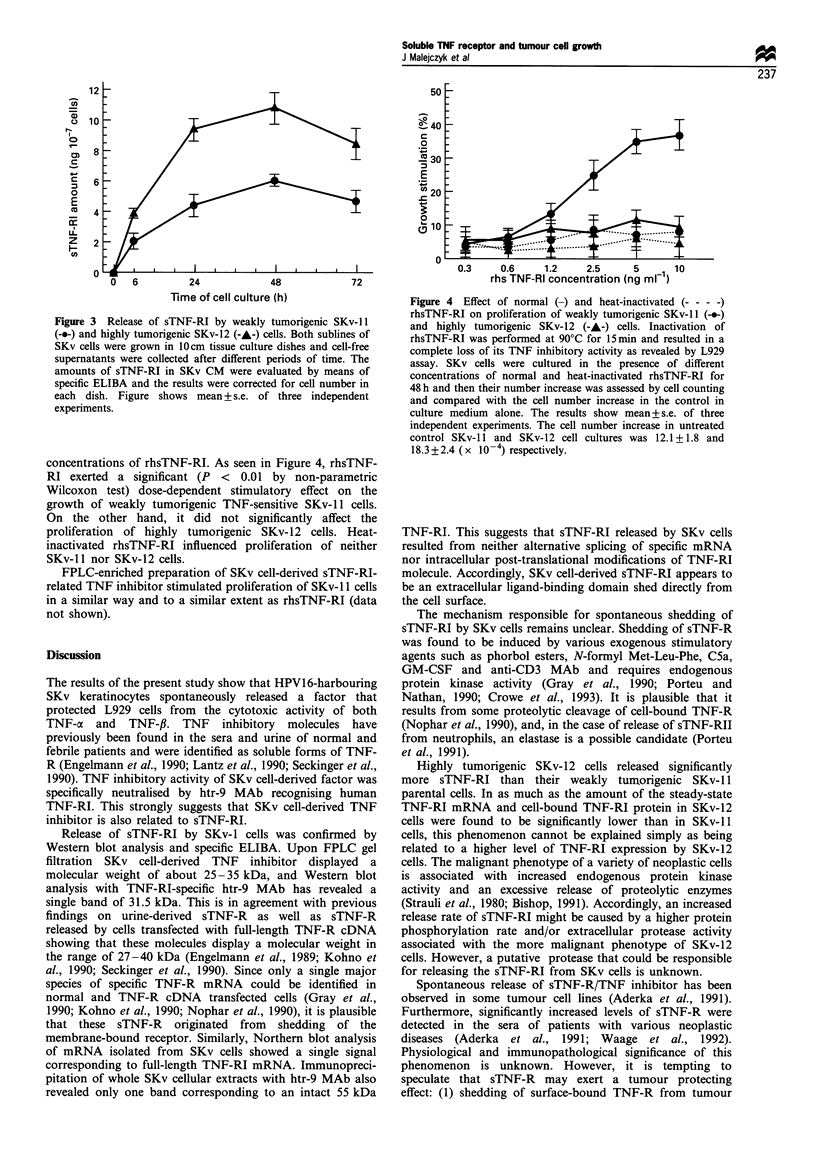
Analysis of conditioned media generated by weakly and highly tumorigenic SKv-1 keratinocyte lines harbouring integrated human papillomavirus type 16 (HPV16) DNA sequences revealed a factor inhibiting TNF-alpha and TNF-beta cytotoxic activity. This inhibitory activity was specifically blocked by htr-9 monoclonal antibody (MAb) recognising 55/60 kDa type I TNF receptor suggesting that it is related to a soluble form of this particular receptor (sTNF-RI). The presence of sTNF-RI was confirmed by Western blot analysis of SKv-1 cell-conditioned medium showing a band of 31.5 kDa as well as by the specific enzyme-linked immunobiological assay (ELIBA). Release of sTNF-RI was a result of shedding because Northern blot analysis showed that SKv-1 cells expressed a full-length TNF-RI mRNA, and radioimmunoprecipitation of TNF-RI from [32S]cysteine-labelled cell extracts demonstrated the presence of normal 55 kDa molecule. Evaluation by ELIBA showed that highly tumorigenic SKv-12 cells released significantly more sTNF-RI than their weakly tumorigenic SKv-11 parental cells. Furthermore, human recombinant as well as SKv cell-derived sTNF-RI stimulated proliferation of weakly tumorigenic SKv-11 cells. This suggests that a progressive growth of some neoplastic cells may be, at least partially, a result of an increased spontaneous release of sTNF-RI that enables the cells to escape from local TNF-alpha-mediated growth inhibition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Englemann H., Hornik V., Skornick Y., Levo Y., Wallach D., Kushtai G. Increased serum levels of soluble receptors for tumor necrosis factor in cancer patients. Cancer Res. 1991 Oct 15;51(20):5602–5607. [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Braun L., Dürst M., Mikumo R., Gruppuso P. Differential response of nontumorigenic and tumorigenic human papillomavirus type 16-positive epithelial cells to transforming growth factor beta 1. Cancer Res. 1990 Nov 15;50(22):7324–7332. [PubMed] [Google Scholar]
- Brockhaus M., Schoenfeld H. J., Schlaeger E. J., Hunziker W., Lesslauer W., Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3127–3131. doi: 10.1073/pnas.87.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe P. D., VanArsdale T. L., Goodwin R. G., Ware C. F. Specific induction of 80-kDa tumor necrosis factor receptor shedding in T lymphocytes involves the cytoplasmic domain and phosphorylation. J Immunol. 1993 Dec 15;151(12):6882–6890. [PubMed] [Google Scholar]
- Engelmann H., Aderka D., Rubinstein M., Rotman D., Wallach D. A tumor necrosis factor-binding protein purified to homogeneity from human urine protects cells from tumor necrosis factor toxicity. J Biol Chem. 1989 Jul 15;264(20):11974–11980. [PubMed] [Google Scholar]
- Engelmann H., Novick D., Wallach D. Two tumor necrosis factor-binding proteins purified from human urine. Evidence for immunological cross-reactivity with cell surface tumor necrosis factor receptors. J Biol Chem. 1990 Jan 25;265(3):1531–1536. [PubMed] [Google Scholar]
- Gray P. W., Barrett K., Chantry D., Turner M., Feldmann M. Cloning of human tumor necrosis factor (TNF) receptor cDNA and expression of recombinant soluble TNF-binding protein. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7380–7384. doi: 10.1073/pnas.87.19.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M. Role of the human papillomaviruses in human cancer. Cancer Res. 1991 Sep 15;51(18 Suppl):5019s–5022s. [PubMed] [Google Scholar]
- Kiviat N. B., Koutsky L. A. Specific human papillomavirus types as the causal agents of most cervical intraepithelial neoplasia: implications for current views and treatment. J Natl Cancer Inst. 1993 Jun 16;85(12):934–935. doi: 10.1093/jnci/85.12.934. [DOI] [PubMed] [Google Scholar]
- Kohno T., Brewer M. T., Baker S. L., Schwartz P. E., King M. W., Hale K. K., Squires C. H., Thompson R. C., Vannice J. L. A second tumor necrosis factor receptor gene product can shed a naturally occurring tumor necrosis factor inhibitor. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8331–8335. doi: 10.1073/pnas.87.21.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S. M., Carver M. E. Serum-free in vitro bioassay for the detection of tumor necrosis factor. J Immunol Methods. 1986 Nov 6;93(2):201–206. doi: 10.1016/0022-1759(86)90189-4. [DOI] [PubMed] [Google Scholar]
- Lantz M., Gullberg U., Nilsson E., Olsson I. Characterization in vitro of a human tumor necrosis factor-binding protein. A soluble form of a tumor necrosis factor receptor. J Clin Invest. 1990 Nov;86(5):1396–1402. doi: 10.1172/JCI114853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski S., Hunzelmann N., Nischt R., Eckes B., Rudnicka L., Orth G., Krieg T., Jablonska S. TGF beta-1 and TNF alpha expression in the epidermis of patients with epidermodysplasia verruciformis. J Invest Dermatol. 1991 Nov;97(5):862–867. doi: 10.1111/1523-1747.ep12491539. [DOI] [PubMed] [Google Scholar]
- Malejczyk J., Malejczyk M., Köck A., Urbanski A., Majewski S., Hunzelmann N., Jablonska S., Orth G., Luger T. A. Autocrine growth limitation of human papillomavirus type 16-harboring keratinocytes by constitutively released tumor necrosis factor-alpha. J Immunol. 1992 Oct 15;149(8):2702–2708. [PubMed] [Google Scholar]
- Malejczyk J., Malejczyk M., Majewski S., Breitburd F., Luger T. A., Jablonska S., Orth G. Increased tumorigenicity of human keratinocytes harboring human papillomavirus type 16 is associated with resistance to endogenous tumor necrosis factor-alpha-mediated growth limitation. Int J Cancer. 1994 Feb 15;56(4):593–598. doi: 10.1002/ijc.2910560421. [DOI] [PubMed] [Google Scholar]
- Malejczyk J., Malejczyk M., Urbanski A., Köck A., Jablonska S., Orth G., Luger T. A. Constitutive release of IL6 by human papillomavirus type 16 (HPV16)-harboring keratinocytes: a mechanism augmenting the NK-cell-mediated lysis of HPV-bearing neoplastic cells. Cell Immunol. 1991 Aug;136(1):155–164. doi: 10.1016/0008-8749(91)90390-w. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Guénet J. L., Buckingham M. E. Number and organization of actin-related sequences in the mouse genome. J Mol Biol. 1983 Jun 15;167(1):77–101. doi: 10.1016/s0022-2836(83)80035-7. [DOI] [PubMed] [Google Scholar]
- Nophar Y., Kemper O., Brakebusch C., Englemann H., Zwang R., Aderka D., Holtmann H., Wallach D. Soluble forms of tumor necrosis factor receptors (TNF-Rs). The cDNA for the type I TNF-R, cloned using amino acid sequence data of its soluble form, encodes both the cell surface and a soluble form of the receptor. EMBO J. 1990 Oct;9(10):3269–3278. doi: 10.1002/j.1460-2075.1990.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteu F., Brockhaus M., Wallach D., Engelmann H., Nathan C. F. Human neutrophil elastase releases a ligand-binding fragment from the 75-kDa tumor necrosis factor (TNF) receptor. Comparison with the proteolytic activity responsible for shedding of TNF receptors from stimulated neutrophils. J Biol Chem. 1991 Oct 5;266(28):18846–18853. [PubMed] [Google Scholar]
- Porteu F., Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. 1990 Aug 1;172(2):599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre-Garau X., Schneider-Maunoury S., Couturier J., Orth G. Human papillomavirus type 16 DNA is integrated into chromosome region 12q14-q15 in a cell line derived from a vulvar intraepithelial neoplasia. Cancer Genet Cytogenet. 1990 Feb;44(2):243–251. doi: 10.1016/0165-4608(90)90053-d. [DOI] [PubMed] [Google Scholar]
- Schneider-Maunoury S., Croissant O., Orth G. Integration of human papillomavirus type 16 DNA sequences: a possible early event in the progression of genital tumors. J Virol. 1987 Oct;61(10):3295–3298. doi: 10.1128/jvi.61.10.3295-3298.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Maunoury S., Pehau-Arnaudet G., Breitburd F., Orth G. Expression of the human papillomavirus type 16 genome in SK-v cells, a line derived from a vulvar intraepithelial neoplasia. J Gen Virol. 1990 Apr;71(Pt 4):809–817. doi: 10.1099/0022-1317-71-4-809. [DOI] [PubMed] [Google Scholar]
- Seckinger P., Zhang J. H., Hauptmann B., Dayer J. M. Characterization of a tumor necrosis factor alpha (TNF-alpha) inhibitor: evidence of immunological cross-reactivity with the TNF receptor. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5188–5192. doi: 10.1073/pnas.87.13.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Waage A., Liabakk N., Lien E., Lamvik J., Espevik T. p55 and p75 tumor necrosis factor receptors in patients with chronic lymphocytic leukemia. Blood. 1992 Nov 15;80(10):2577–2583. [PubMed] [Google Scholar]
- Woodworth C. D., Notario V., DiPaolo J. A. Transforming growth factors beta 1 and 2 transcriptionally regulate human papillomavirus (HPV) type 16 early gene expression in HPV-immortalized human genital epithelial cells. J Virol. 1990 Oct;64(10):4767–4775. doi: 10.1128/jvi.64.10.4767-4775.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989 Sep 1;49(17):4677–4681. [PubMed] [Google Scholar]




