Abstract
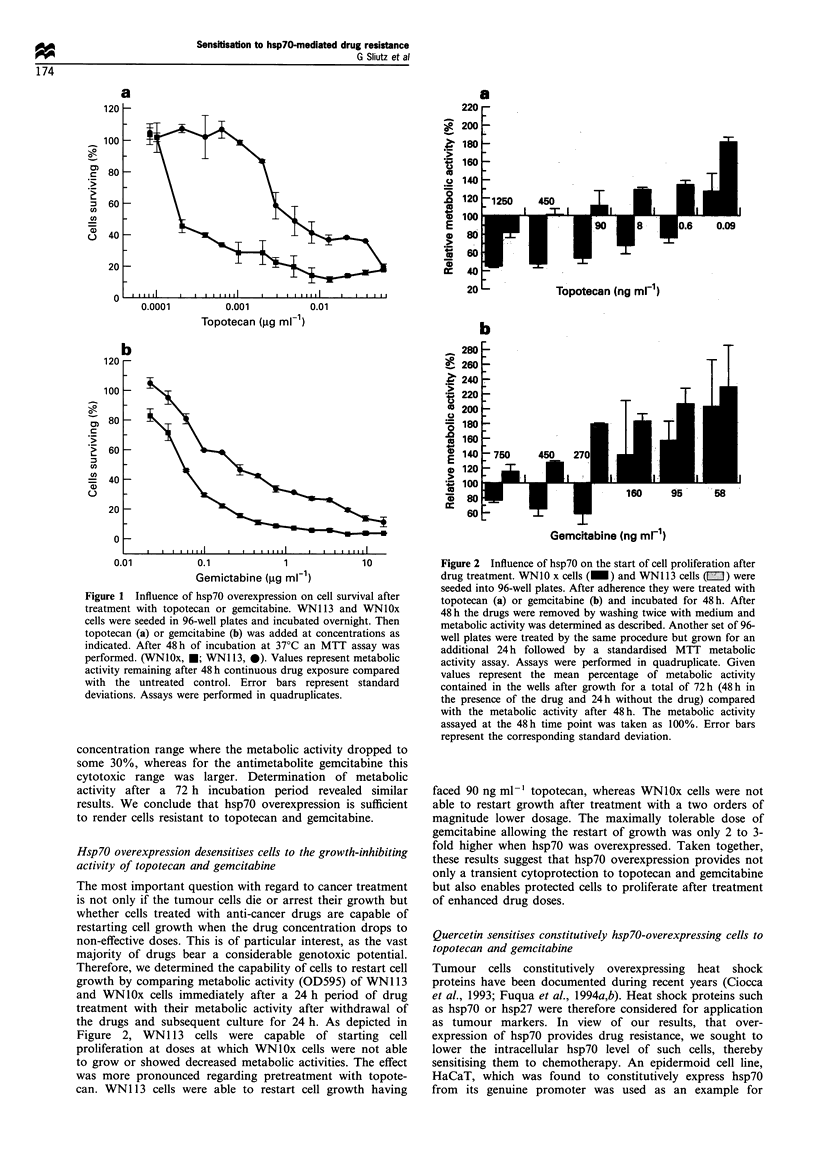
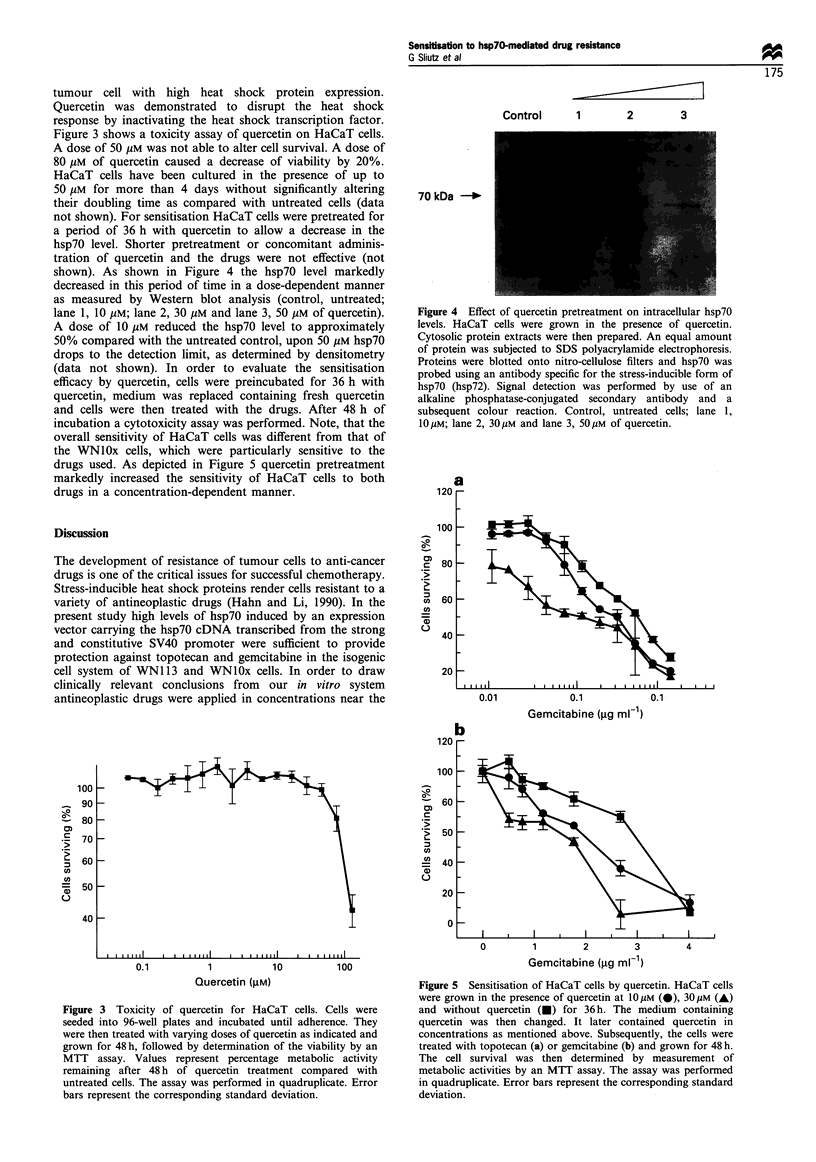
Heat shock proteins have been reported to confer resistance to certain antineoplastic drugs. We investigated the impact of hsp70 overexpression on the efficacy of two new anti-cancer drugs, topotecan and gemcitabine. We used the fibrosarcoma WEHI-S cells stably transfected to overexpress the hsp70 cDNA from the constitutive SV40 promoter and appropriate control cells. After topotecan and gemcitabine treatment hsp70-overexpressing cells showed a marked elevation in cell survival, suggesting that hsp70 overexpression was sufficient to confer resistance to the drugs. In addition, hsp70-overexpressing cells were capable of starting cell proliferation after treatment with drug dosages that were lethal to control cells. Our results demonstrate that hsp70 overexpression represents a possible cause of drug resistance. In order to transfer these data to tumour cells constitutively expressing stress hsp70 due to the constitutive activity of the original hsp70 promoter we sought to supress the heat shock response pathway by the natural flavonoid quercetin, known to inactivate the heat shock transcription factor (HSF). Using a suitable cell line, we demonstrated the sensitising activity of quercetin. We found that antineoplastic drug concentrations exerting cytotoxic activity were markedly lower when cells were pretreated with quercetin. Concomitantly, hsp70 expression was strongly down-regulated under quercetin treatment. Our data indicate that quercetin may be useful as a sensitiser in chemotherapeutically treated patients suffering from hsp70-overexpressing tumours.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boukamp P., Stanbridge E. J., Foo D. Y., Cerutti P. A., Fusenig N. E. c-Ha-ras oncogene expression in immortalized human keratinocytes (HaCaT) alters growth potential in vivo but lacks correlation with malignancy. Cancer Res. 1990 May 1;50(9):2840–2847. [PubMed] [Google Scholar]
- Breitkreutz D., Boukamp P., Ryle C. M., Stark H. J., Roop D. R., Fusenig N. E. Epidermal morphogenesis and keratin expression in c-Ha-ras-transfected tumorigenic clones of the human HaCaT cell line. Cancer Res. 1991 Aug 15;51(16):4402–4409. [PubMed] [Google Scholar]
- Burke T. G., Mi Z. The structural basis of camptothecin interactions with human serum albumin: impact on drug stability. J Med Chem. 1994 Jan 7;37(1):40–46. doi: 10.1021/jm00027a005. [DOI] [PubMed] [Google Scholar]
- Ciocca D. R., Clark G. M., Tandon A. K., Fuqua S. A., Welch W. J., McGuire W. L. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst. 1993 Apr 7;85(7):570–574. doi: 10.1093/jnci/85.7.570. [DOI] [PubMed] [Google Scholar]
- Elledge R. M., Clark G. M., Fuqua S. A., Yu Y. Y., Allred D. C. p53 protein accumulation detected by five different antibodies: relationship to prognosis and heat shock protein 70 in breast cancer. Cancer Res. 1994 Jul 15;54(14):3752–3757. [PubMed] [Google Scholar]
- Fujita F., Fujita M., Fujita M., Sakamoto Y. [Acquired resistance and cross-resistance of gemcitabine to cisplatin or vindesine in human lung cancer xenografted in nude mice]. Gan To Kagaku Ryoho. 1994 Dec;21(16):2749–2755. [PubMed] [Google Scholar]
- Fujita M., Fujita F., Inaba H., Taguchi T. [Antitumor activity of LY 188011, a new deoxycytidine analog, against human cancers xenografted into nude mice]. Gan To Kagaku Ryoho. 1994 Mar;21(4):517–523. [PubMed] [Google Scholar]
- Fuqua S. A., Benedix M. G., Krieg S., Weng C. N., Chamness G. C., Oesterreich S. Constitutive overexpression of the 27,000 dalton heat shock protein in late passage human breast cancer cells. Breast Cancer Res Treat. 1994;32(2):177–186. doi: 10.1007/BF00665768. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Oesterreich S., Hilsenbeck S. G., Von Hoff D. D., Eckardt J., Osborne C. K. Heat shock proteins and drug resistance. Breast Cancer Res Treat. 1994;32(1):67–71. doi: 10.1007/BF00666207. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Hosokawa N., Hirayoshi K., Kudo H., Takechi H., Aoike A., Kawai K., Nagata K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol Cell Biol. 1992 Aug;12(8):3490–3498. doi: 10.1128/mcb.12.8.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jättelä M., Wissing D., Bauer P. A., Li G. C. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992 Oct;11(10):3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J. S., Schuetz T. J., Kingston R. E. Activation in vitro of sequence-specific DNA binding by a human regulatory factor. Nature. 1988 Sep 22;335(6188):372–375. doi: 10.1038/335372a0. [DOI] [PubMed] [Google Scholar]
- Lee Y. J., Erdos G., Hou Z. Z., Kim S. H., Kim J. H., Cho J. M., Corry P. M. Mechanism of quercetin-induced suppression and delay of heat shock gene expression and thermotolerance development in HT-29 cells. Mol Cell Biochem. 1994 Aug 31;137(2):141–154. doi: 10.1007/BF00944076. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Nagai N., Nakai A., Nagata K. Quercetin suppresses heat shock response by down regulation of HSF1. Biochem Biophys Res Commun. 1995 Mar 28;208(3):1099–1105. doi: 10.1006/bbrc.1995.1447. [DOI] [PubMed] [Google Scholar]
- Oesterreich S., Weng C. N., Qiu M., Hilsenbeck S. G., Osborne C. K., Fuqua S. A. The small heat shock protein hsp27 is correlated with growth and drug resistance in human breast cancer cell lines. Cancer Res. 1993 Oct 1;53(19):4443–4448. [PubMed] [Google Scholar]
- Ruiz van Haperen V. W., Peters G. J. New targets for pyrimidine antimetabolites for the treatment of solid tumours. 2: Deoxycytidine kinase. Pharm World Sci. 1994 Apr 15;16(2):104–112. doi: 10.1007/BF01880661. [DOI] [PubMed] [Google Scholar]
- Schröder H., Langer T., Hartl F. U., Bukau B. DnaK, DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J. 1993 Nov;12(11):4137–4144. doi: 10.1002/j.1460-2075.1993.tb06097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. M., Aragane Y., Schwarz A., Luger T. A., Schwarz T. UVB light induces nuclear factor kappa B (NF kappa B) activity independently from chromosomal DNA damage in cell-free cytosolic extracts. J Invest Dermatol. 1994 Apr;102(4):422–427. doi: 10.1111/1523-1747.ep12372194. [DOI] [PubMed] [Google Scholar]
- Simon M. M., Reikerstorfer A., Schwarz A., Krone C., Luger T. A., Jättelä M., Schwarz T. Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. J Clin Invest. 1995 Mar;95(3):926–933. doi: 10.1172/JCI117800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B. K. Topoisomerase inhibitors. A review of their therapeutic potential in cancer. Drugs. 1995 Jan;49(1):11–19. doi: 10.2165/00003495-199549010-00002. [DOI] [PubMed] [Google Scholar]
- Skowyra D., Georgopoulos C., Zylicz M. The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell. 1990 Sep 7;62(5):939–944. doi: 10.1016/0092-8674(90)90268-j. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Watanabe M. Modulation of cell growth and mutation induction by introduction of the expression vector of human hsp70 gene. Exp Cell Res. 1994 Nov;215(1):75–81. doi: 10.1006/excr.1994.1317. [DOI] [PubMed] [Google Scholar]



