Abstract
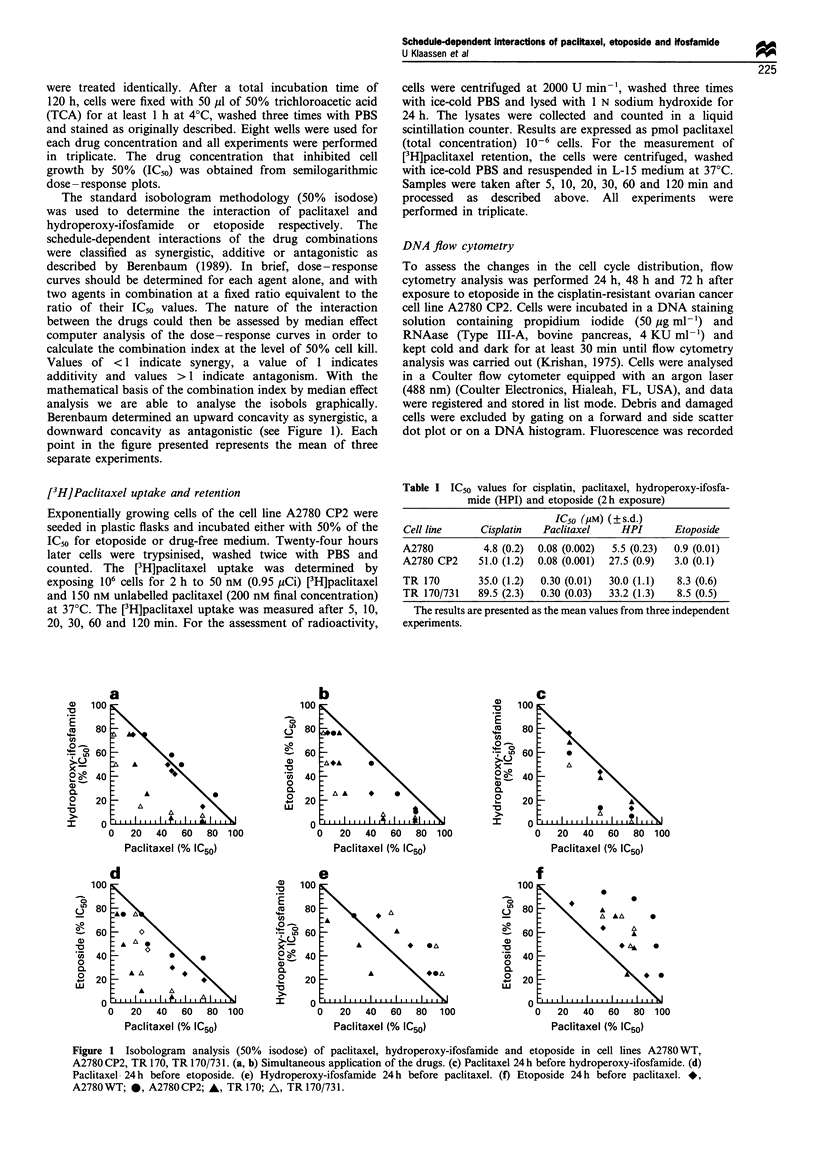
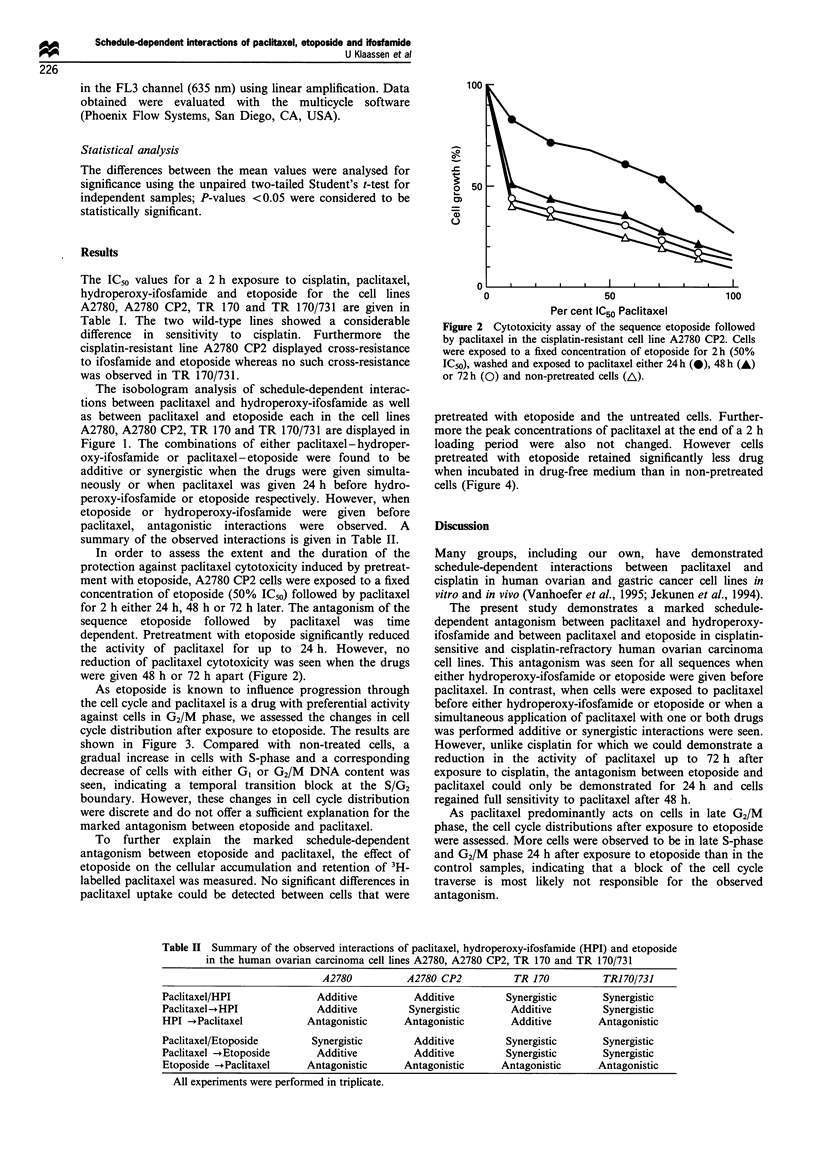
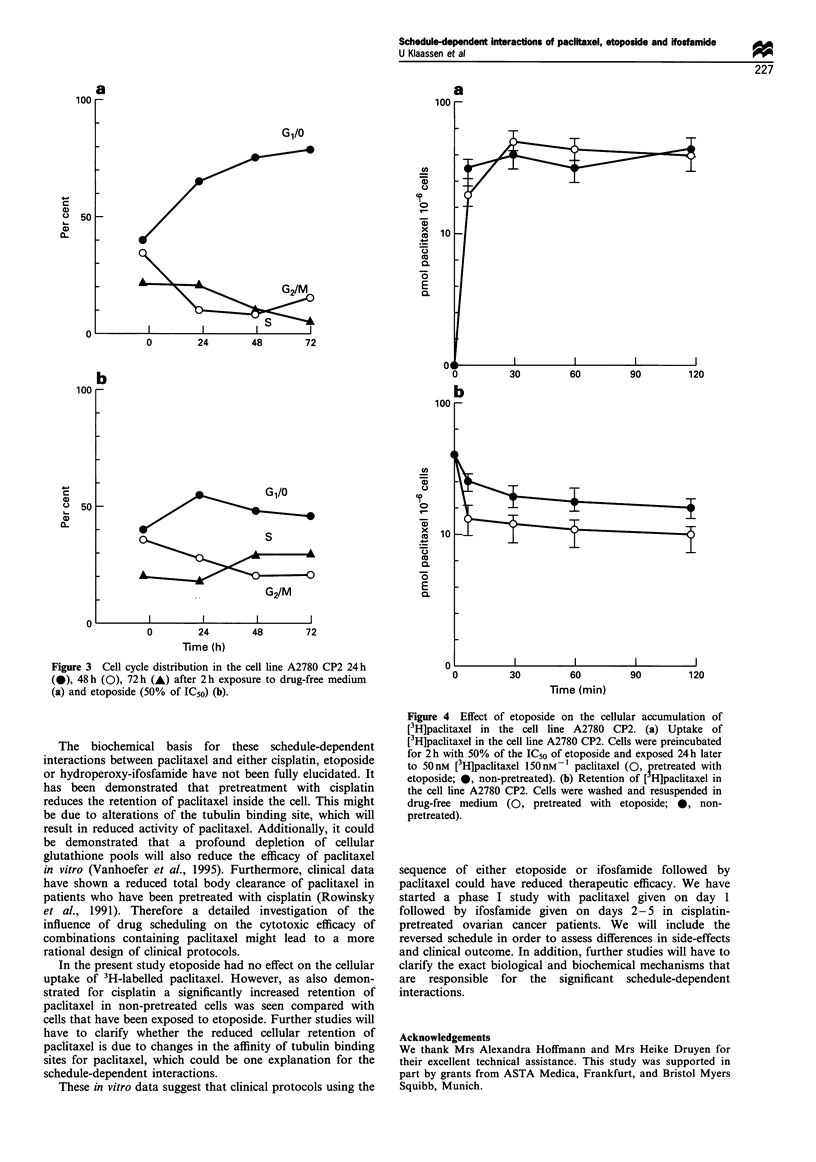
Paclitaxel has demonstrated broad clinical activity in a variety of malignancies both alone and in combination with other chemotherapeutic agents. The in vitro cytotoxicity of a 2 h exposure to paclitaxel, hydroperoxy-ifosfamide and etoposide alone, in combination and in sequence, was evaluated against established cisplatin-sensitive and cisplatin-refractory human ovarian carcinoma cell lines using isobologram analysis. The combinations of either paclitaxel-hydroperoxy-ifosfamide or paclitaxel-etoposide were found to be additive or synergistic when the drugs were given simultaneously or when paclitaxel was given 24 h before hydroperoxy-ifosfamide or etoposide respectively. However, when etoposide or hydroperoxy-ifosfamide were given before paclitaxel, antagonistic interactions were observed. With regard to etoposide this antagonism was evident for up to 24 h. In agreement with our data with the schedule-dependent interactions of paclitaxel and cisplatin in human gastric and ovarian carcinoma cell lines, these data demonstrate that the interactions of paclitaxel, etoposide and hydroperoxy-ifosfamide are also highly schedule dependent and applications of etoposide or ifosfamide before paclitaxel may result in pronounced antagonism. These findings could have implications for the design of further clinical protocols.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hamilton T. C., Winker M. A., Louie K. G., Batist G., Behrens B. C., Tsuruo T., Grotzinger K. R., McKoy W. M., Young R. C., Ozols R. F. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol. 1985 Jul 15;34(14):2583–2586. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- Jekunen A. P., Christen R. D., Shalinsky D. R., Howell S. B. Synergistic interaction between cisplatin and taxol in human ovarian carcinoma cells in vitro. Br J Cancer. 1994 Feb;69(2):299–306. doi: 10.1038/bjc.1994.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Ozols R. F., Lai G. M., Fojo A., Rothenberg M., Hamilton T. C. Increased DNA repair as a mechanism of acquired resistance to cis-diamminedichloroplatinum (II) in human ovarian cancer cell lines. Cancer Res. 1988 Oct 15;48(20):5713–5716. [PubMed] [Google Scholar]
- Rogan A. M., Hamilton T. C., Young R. C., Klecker R. W., Jr, Ozols R. F. Reversal of adriamycin resistance by verapamil in human ovarian cancer. Science. 1984 Jun 1;224(4652):994–996. doi: 10.1126/science.6372095. [DOI] [PubMed] [Google Scholar]
- Rowinsky E. K., Gilbert M. R., McGuire W. P., Noe D. A., Grochow L. B., Forastiere A. A., Ettinger D. S., Lubejko B. G., Clark B., Sartorius S. E. Sequences of taxol and cisplatin: a phase I and pharmacologic study. J Clin Oncol. 1991 Sep;9(9):1692–1703. doi: 10.1200/JCO.1991.9.9.1692. [DOI] [PubMed] [Google Scholar]
- Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990 Jul 4;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Thigpen J. T., Blessing J. A., Ball H., Hummel S. J., Barrett R. J. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: a Gynecologic Oncology Group study. J Clin Oncol. 1994 Sep;12(9):1748–1753. doi: 10.1200/JCO.1994.12.9.1748. [DOI] [PubMed] [Google Scholar]
- Vanhoefer U., Harstrick A., Wilke H., Schleucher N., Walles H., Schröder J., Seeber S. Schedule-dependent antagonism of paclitaxel and cisplatin in human gastric and ovarian carcinoma cell lines in vitro. Eur J Cancer. 1995;31A(1):92–97. doi: 10.1016/0959-8049(94)00440-g. [DOI] [PubMed] [Google Scholar]



