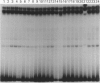
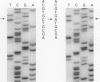
Abstract
Cyclin-dependent kinase-4 inhibitor gene (p16INK4) has recently been mapped to chromosome 9p21. Homozygous deletions of this gene have been found at high frequency in cell lines derived from different types of tumours. These findings suggested therefore, that p16INK4 is a tumour-suppressor gene involved in a wide variety of human cancers. To investigate the frequency of p16INK mutations/deletions in prostate cancer, we screened 20 primary prostate tumours and four established cell lines by polymerase chain reaction (PCR) and single-strand conformation polymorphism (SSCP) analysis for exon 1 and exon 2. In contrast to most previous reports, no homozygous deletions were found in prostate cancer cell lines, but one cell line (DU145) has revealed to a mutation at codon 76. Only two SSCP shifts were detected in primary tumours: one of them corresponds to a mutation at codon 55 and the other one probably corresponds to a polymorphism. These data suggest that mutation of the p16INK4 gene is not a frequent genetic alteration implicated in prostate cancer development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergerheim U. S., Kunimi K., Collins V. P., Ekman P. Deletion mapping of chromosomes 8, 10, and 16 in human prostatic carcinoma. Genes Chromosomes Cancer. 1991 May;3(3):215–220. doi: 10.1002/gcc.2870030308. [DOI] [PubMed] [Google Scholar]
- Bova G. S., Carter B. S., Bussemakers M. J., Emi M., Fujiwara Y., Kyprianou N., Jacobs S. C., Robinson J. C., Epstein J. I., Walsh P. C. Homozygous deletion and frequent allelic loss of chromosome 8p22 loci in human prostate cancer. Cancer Res. 1993 Sep 1;53(17):3869–3873. [PubMed] [Google Scholar]
- Cairns P., Mao L., Merlo A., Lee D. J., Schwab D., Eby Y., Tokino K., van der Riet P., Blaugrund J. E., Sidransky D. Rates of p16 (MTS1) mutations in primary tumors with 9p loss. Science. 1994 Jul 15;265(5170):415–417. doi: 10.1126/science.8023167. [DOI] [PubMed] [Google Scholar]
- Cheng J. Q., Jhanwar S. C., Klein W. M., Bell D. W., Lee W. C., Altomare D. A., Nobori T., Olopade O. I., Buckler A. J., Testa J. R. p16 alterations and deletion mapping of 9p21-p22 in malignant mesothelioma. Cancer Res. 1994 Nov 1;54(21):5547–5551. [PubMed] [Google Scholar]
- Hannon G. J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994 Sep 15;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Jacoby L. B., MacCollin M., Louis D. N., Mohney T., Rubio M. P., Pulaski K., Trofatter J. A., Kley N., Seizinger B., Ramesh V. Exon scanning for mutation of the NF2 gene in schwannomas. Hum Mol Genet. 1994 Mar;3(3):413–419. doi: 10.1093/hmg/3.3.413. [DOI] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T., Miura K., Aoki T., Nishihira T., Mori S., Nakamura Y. Frequent somatic mutation of the MTS1/CDK4I (multiple tumor suppressor/cyclin-dependent kinase 4 inhibitor) gene in esophageal squamous cell carcinoma. Cancer Res. 1994 Jul 1;54(13):3396–3397. [PubMed] [Google Scholar]
- Nasmyth K., Hunt T. Cell cycle. Dams and sluices. Nature. 1993 Dec 16;366(6456):634–635. doi: 10.1038/366634a0. [DOI] [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Phillips S. M., Barton C. M., Lee S. J., Morton D. G., Wallace D. M., Lemoine N. R., Neoptolemos J. P. Loss of the retinoblastoma susceptibility gene (RB1) is a frequent and early event in prostatic tumorigenesis. Br J Cancer. 1994 Dec;70(6):1252–1257. doi: 10.1038/bjc.1994.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994 Jan;8(1):9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Beck J. S., Kwitek A. E., Sandstrom D. W., Stone E. M. The sensitivity of single-strand conformation polymorphism analysis for the detection of single base substitutions. Genomics. 1993 May;16(2):325–332. doi: 10.1006/geno.1993.1193. [DOI] [PubMed] [Google Scholar]
- Spruck C. H., 3rd, Gonzalez-Zulueta M., Shibata A., Simoneau A. R., Lin M. F., Gonzales F., Tsai Y. C., Jones P. A. p16 gene in uncultured tumours. Nature. 1994 Jul 21;370(6486):183–184. doi: 10.1038/370183a0. [DOI] [PubMed] [Google Scholar]
- Ueki K., Rubio M. P., Ramesh V., Correa K. M., Rutter J. L., von Deimling A., Buckler A. J., Gusella J. F., Louis D. N. MTS1/CDKN2 gene mutations are rare in primary human astrocytomas with allelic loss of chromosome 9p. Hum Mol Genet. 1994 Oct;3(10):1841–1845. doi: 10.1093/hmg/3.10.1841. [DOI] [PubMed] [Google Scholar]
- Visakorpi T., Kallioniemi A. H., Syvänen A. C., Hyytinen E. R., Karhu R., Tammela T., Isola J. J., Kallioniemi O. P. Genetic changes in primary and recurrent prostate cancer by comparative genomic hybridization. Cancer Res. 1995 Jan 15;55(2):342–347. [PubMed] [Google Scholar]
- Xu L., Sgroi D., Sterner C. J., Beauchamp R. L., Pinney D. M., Keel S., Ueki K., Rutter J. L., Buckler A. J., Louis D. N. Mutational analysis of CDKN2 (MTS1/p16ink4) in human breast carcinomas. Cancer Res. 1994 Oct 15;54(20):5262–5264. [PubMed] [Google Scholar]
- du Manoir S., Speicher M. R., Joos S., Schröck E., Popp S., Döhner H., Kovacs G., Robert-Nicoud M., Lichter P., Cremer T. Detection of complete and partial chromosome gains and losses by comparative genomic in situ hybridization. Hum Genet. 1993 Feb;90(6):590–610. doi: 10.1007/BF00202476. [DOI] [PubMed] [Google Scholar]