Abstract
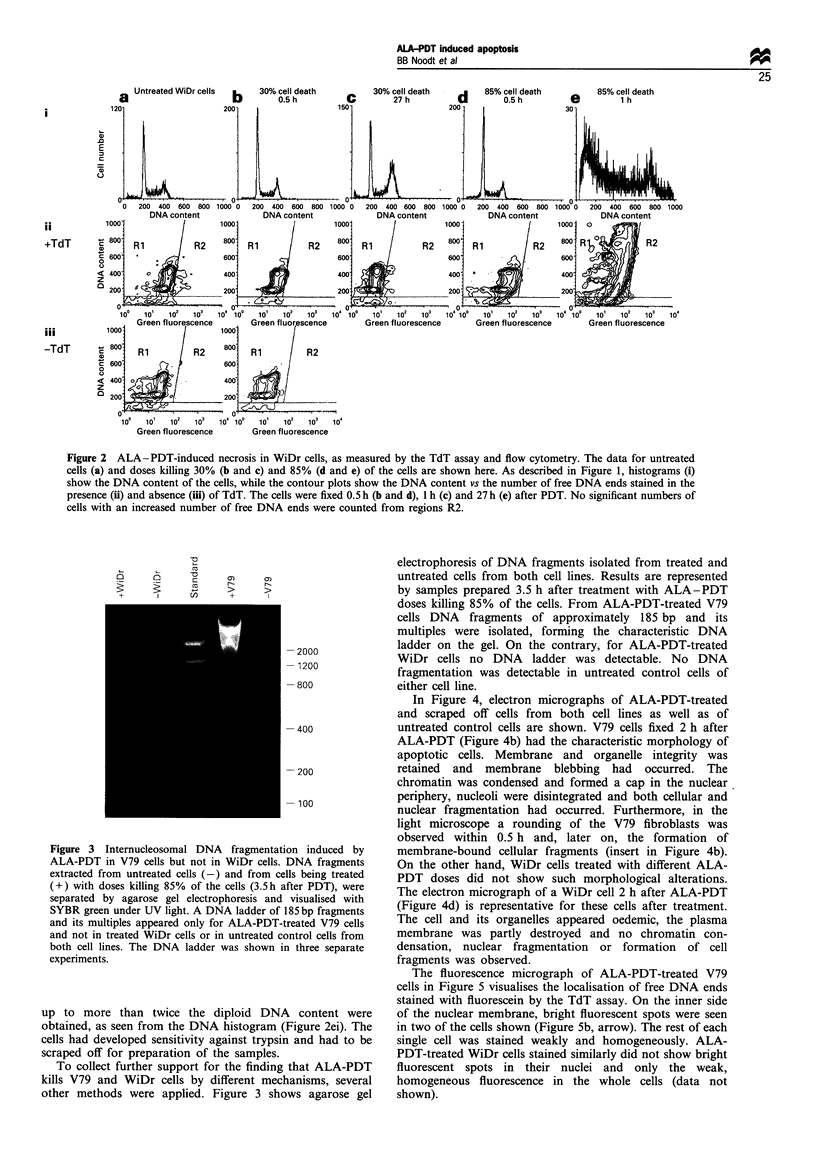
The mode of cell death induced by photodynamic treatment (PDT) was studied in two cell lines cultured in monolayer, V79 Chinese hamster fibroblasts and WiDr human colon adenocarcinoma cells. The cells were incubated with 5-aminolaevulinic acid (5-ALA) as a precursor for the endogenously synthesised protoporphyrin IX, which was activated by light. Free DNA ends, owing to internucleosomal DNA cleavage in apoptotic cells, were stained specifically with a fluorescent dye in the terminal deoxynucleotidyl transferase (TdT) assay. The free DNA ends were measured by flow cytometry and the fractions of apoptotic cells determined. Total cell death was measured in a cell survival assay to determine the necrotic fraction after subtraction of the apoptotic fraction. V79 cells did undergo apoptosis while WiDr cells were killed only through necrosis. With time, the apoptotic fraction of V79 cells increased until a maximum was reached about 3-4 h after ALA-PDT treatment. For increasing ALA-PDT doses, a maximal apoptotic fraction 75-85% of the cells was measured at about 85% of total cell death. The flow cytometric assay of apoptosis was confirmed by the typical ladder of oligonucleosomal DNA fragments obtained from agarose gel electrophoresis, by fluorescence micrographs visualising the induced free DNA ends and by electron micrographs showing the typical morphology of apoptotic cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal M. L., Clay M. E., Harvey E. J., Evans H. H., Antunez A. R., Oleinick N. L. Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells. Cancer Res. 1991 Nov 1;51(21):5993–5996. [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur E., Dubbelman T. M., Van Steveninck J. Phthalocyanine-induced photodynamic changes of cytoplasmic free calcium in Chinese hamster cells. Photochem Photobiol. 1991 Aug;54(2):163–166. doi: 10.1111/j.1751-1097.1991.tb02002.x. [DOI] [PubMed] [Google Scholar]
- Ben-Hur E., Rosenthal I. The phthalocyanines: a new class of mammalian cells photosensitizers with a potential for cancer phototherapy. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Feb;47(2):145–147. doi: 10.1080/09553008514550211. [DOI] [PubMed] [Google Scholar]
- Berg K., Luksiene Z., Moan J., Ma L. Combined treatment of ionizing radiation and photosensitization by 5-aminolevulinic acid-induced protoporphyrin IX. Radiat Res. 1995 Jun;142(3):340–346. [PubMed] [Google Scholar]
- Berg K., Madslien K., Bommer J. C., Oftebro R., Winkelman J. W., Moan J. Light induced relocalization of sulfonated meso-tetraphenylporphines in NHIK 3025 cells and effects of dose fractionation. Photochem Photobiol. 1991 Feb;53(2):203–210. doi: 10.1111/j.1751-1097.1991.tb03924.x. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Ribeiro J. M. Apoptosis and disease. Lancet. 1993 May 15;341(8855):1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Smith A. Differential interaction of porphyrins used in photoradiation therapy with ferrochelatase. Biochem J. 1984 Oct 15;223(2):441–445. doi: 10.1042/bj2230441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Li X., Gong J. Assays of cell viability: discrimination of cells dying by apoptosis. Methods Cell Biol. 1994;41:15–38. doi: 10.1016/s0091-679x(08)61707-0. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J., Kaufman J. E., Goldfarb A., Weishaupt K. R., Boyle D., Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978 Aug;38(8):2628–2635. [PubMed] [Google Scholar]
- Dougherty T. J. Photodynamic therapy. Photochem Photobiol. 1993 Dec;58(6):895–900. doi: 10.1111/j.1751-1097.1993.tb04990.x. [DOI] [PubMed] [Google Scholar]
- Fisher D. E. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994 Aug 26;78(4):539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- Gantchev T. G. Spin-trapping of free radicals during phthalocyanine photosensitization of lymphoma cells in vitro. Cancer Biochem Biophys. 1992 Nov;13(2):103–111. [PubMed] [Google Scholar]
- Gaullier J. M., Gèze M., Santus R., Sa e Melo T., Mazière J. C., Bazin M., Morlière P., Dubertret L. Subcellular localization of and photosensitization by protoporphyrin IXhuman keratinocytes and fibroblasts cultivated with 5-aminolevulinic acid. Photochem Photobiol. 1995 Jul;62(1):114–122. doi: 10.1111/j.1751-1097.1995.tb05247.x. [DOI] [PubMed] [Google Scholar]
- Gorczyca W., Gong J., Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993 Apr 15;53(8):1945–1951. [PubMed] [Google Scholar]
- He X. Y., Sikes R. A., Thomsen S., Chung L. W., Jacques S. L. Photodynamic therapy with photofrin II induces programmed cell death in carcinoma cell lines. Photochem Photobiol. 1994 Apr;59(4):468–473. doi: 10.1111/j.1751-1097.1994.tb05066.x. [DOI] [PubMed] [Google Scholar]
- Herrmann M., Lorenz H. M., Voll R., Grünke M., Woith W., Kalden J. R. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994 Dec 11;22(24):5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. C., Pottier R. H., Pross D. C. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B. 1990 Jun;6(1-2):143–148. doi: 10.1016/1011-1344(90)85083-9. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvam E., Moan J. A comparison of three photosensitizers with respect to efficiency of cell inactivation, fluorescence quantum yield and DNA strand breaks. Photochem Photobiol. 1990 Oct;52(4):769–773. doi: 10.1111/j.1751-1097.1990.tb08680.x. [DOI] [PubMed] [Google Scholar]
- Kvam E., Stokke T., Moan J. The lengths of DNA fragments light-induced in the presence of a photosensitizer localized at the nuclear membrane of human cells. Biochim Biophys Acta. 1990 May 24;1049(1):33–37. doi: 10.1016/0167-4781(90)90081-c. [DOI] [PubMed] [Google Scholar]
- Lennon S. V., Martin S. J., Cotter T. G. Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif. 1991 Mar;24(2):203–214. doi: 10.1111/j.1365-2184.1991.tb01150.x. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Malik Z., Lugaci H. Destruction of erythroleukaemic cells by photoactivation of endogenous porphyrins. Br J Cancer. 1987 Nov;56(5):589–595. doi: 10.1038/bjc.1987.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J., Green D. R., Cotter T. G. Dicing with death: dissecting the components of the apoptosis machinery. Trends Biochem Sci. 1994 Jan;19(1):26–30. doi: 10.1016/0968-0004(94)90170-8. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Moan J., Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem Photobiol. 1991 Apr;53(4):549–553. doi: 10.1111/j.1751-1097.1991.tb03669.x. [DOI] [PubMed] [Google Scholar]
- Moan J., Pettersen E. O., Christensen T. The mechanism of photodynamic inactivation of human cells in vitro in the presence of haematoporphyrin. Br J Cancer. 1979 Apr;39(4):398–407. doi: 10.1038/bjc.1979.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moan J., Waksvik H., Christensen T. DNA single-strand breaks and sister chromatid exchanges induced by treatment with hematoporphyrin and light or by x-rays in human NHIK 3025 cells. Cancer Res. 1980 Aug;40(8 Pt 1):2915–2918. [PubMed] [Google Scholar]
- Noguchi P., Wallace R., Johnson J., Earley E. M., O'Brien S., Ferrone S., Pellegrino M. A., Milstien J., Needy C., Browne W. Characterization of the WIDR: a human colon carcinoma cell line. In Vitro. 1979 Jun;15(6):401–408. doi: 10.1007/BF02618407. [DOI] [PubMed] [Google Scholar]
- Noodt B. B., Kvam E., Steen H. B., Moan J. Primary DNA damage, HPRT mutation and cell inactivation photoinduced with various sensitizers in V79 cells. Photochem Photobiol. 1993 Oct;58(4):541–547. doi: 10.1111/j.1751-1097.1993.tb04929.x. [DOI] [PubMed] [Google Scholar]
- Penning L. C., Lagerberg J. W., VanDierendonck J. H., Cornelisse C. J., Dubbelman T. M., VanSteveninck J. The role of DNA damage and inhibition of poly(ADP-ribosyl)ation in loss of clonogenicity of murine L929 fibroblasts, caused by photodynamically induced oxidative stress. Cancer Res. 1994 Nov 1;54(21):5561–5567. [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]
- Wyllie A. H. Apoptosis (the 1992 Frank Rose Memorial Lecture). Br J Cancer. 1993 Feb;67(2):205–208. doi: 10.1038/bjc.1993.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi S. I., Agarwal R., Eichler G., Rihter B. D., Kenney M. E., Mukhtar H. Photodynamic effects of new silicon phthalocyanines: in vitro studies utilizing rat hepatic microsomes and human erythrocyte ghosts as model membrane sources. Photochem Photobiol. 1993 Aug;58(2):204–210. doi: 10.1111/j.1751-1097.1993.tb09550.x. [DOI] [PubMed] [Google Scholar]






