Abstract
The search for microtubule-associated proteins (MAPs) in plants is relatively recent. In particular, the “classical MAPs,” which stimulate the polymerization and stabilization of microtubules, have only been examined in heterogeneous fractions. As a first step in dissecting the role of individual MAPs, we have chromatographically purified a single 60-kDa protein from a carrot MAP fraction and analyzed its effects on tubulin assembly. MAP60 promoted the formation of long, morphologically regular brain microtubules in vitro, an effect inhibited by preincubation of the MAP with affinity-purified antibodies against this protein. MAP60 also increased the stability of microtubules to dilution and significantly enhanced cold stability to the normally cold-sensitive neurotubules. These in vitro properties are consistent with a role for MAP60 in regulating the turnover/assembly of dynamic plant microtubules in vivo.
When microtubules were isolated from mammalian brain by repeated cycles of assembly and disassembly, various nontubulin proteins were copurified as well. It was subsequently realized that the in vitro assembly of tubulin into microtubules was greatly facilitated by the presence of these microtubule-associated proteins (MAPs) and that MAPs are important in stabilizing microtubules (1–3). The classic definition of a MAP thus became a protein that, upon binding to a microtubule, alters its function and/or behavior (4).
The animal MAPs that have been studied most extensively—tau, MAP1, MAP2, and the nonneuronal MAP4—all stimulate the assembly and stability of microtubules in vitro (5, 6). Assembly is promoted by nucleation, often in combination with a lowering of the critical concentration for tubulin polymerization (7). The microtubule-stabilizing effect of these MAPs lies in their ability to alter dynamic instability by suppressing disassembly, causing an overall elongation of the microtubules (8, 9). MAPs can also bring about a bundling of microtubules, although the mechanism of this activity is under scrutiny (10–12).
Plant microtubules are at least as dynamic as animal microtubules (13, 14), and it is reasonable to assume, by analogy with the animal systems, that the dynamics of plant microtubules are also regulated by MAPs. Yet, whereas the understanding of how MAPs function in animal cells is steadily progressing, our knowledge on MAPs in plant cells lags behind. MAP fractions of varying degrees of purification have been found to promote the bundling of taxol-stabilized brain microtubules, to affect tubulin polymerization, and to confer cold stability upon microtubules (15–19). Though this suggests the likely presence of factors that influence the assembly and stabilization of microtubules, it has not yet been possible to ascribe these activities to single proteins.
We recently reported on the isolation from carrot cytoskeletons of a MAP fraction that strongly stimulated microtubule assembly (20). Using affinity-purified antibodies, we were able to show that some of the proteins in this fraction codistributed with all microtubule arrays throughout the cell cycle, making them potential candidates for regulators of microtubule dynamics. Among the proteins identified as such was a group of three with molecular masses of 60, 62, and 68 kDa; these were immunologically related to a group of proteins previously described by Jiang and Sonobe (21) as the 65-kDa proteins. To begin to dissect the functions of individual MAPs, we have purified MAP60. When tested in vitro, this individual protein was found to stimulate microtubule assembly, enhance microtubule stability to dilution, and confer cold stability upon microtubules.
MATERIALS AND METHODS
Purification of MAP60.
All experiments were carried out in 50 mM Pipes/5 mM MgSO4/5 mM EGTA, pH 6.9 (PME buffer). Crude MAP fractions from suspension cells of carrot (Daucus carota L.) were prepared as described earlier (20). The MAP fraction was passed through a 0.2-μm syringe filter before being applied to a fast protein liquid chromotography (FPLC) system (Gilson) equipped with a DEAE 5PW anion exchange column (Anachem, Luton, U.K.) that had been preequilibrated with PME buffer. Unabsorbed materials were washed out with PME buffer, and a 60-ml linear gradient of 0–600 mM NaCl in PME buffer was used to elute proteins. The chromatographic run was performed at room temperature with a flow rate of 1 ml/min. Fractions of 1 ml were collected and characterized by SDS/PAGE. Gel analysis and immunoblots using whole anti-MAP serum and blot affinity-purified antibodies against the 65-kDa MAP family (anti-65 kDa) were performed as described by Chan et al. (20).
Preparation of Neurotubulin.
DEAE-A50-purified tubulin was prepared from pig brain as described in Wymer et al. (22). In polymerization buffer [PB: PME buffer supplemented with 5% (vol/vol) DMSO and GTP to 1 mM] incubated at 37°C, we found that the critical concentration for free nucleation was close to 15 μM (0.75 mg/ml neurotubulin).
Rhodamine Tubulin Assay.
Rhodamine-labeled tubulin was prepared according to Hyman et al. (23). The attachment of label slightly affected the polymerization characteristics. For our rhodamine-conjugated tubulin, the critical concentration for self-assembly, analyzed over a 2-h period, was nearly 20 μM. Polymerization assays were carried out in Eppendorf tubes in final volumes of 5 or 10 μl. All solutions were in PB and were kept on ice for 10 min after mixing. The assay was started by transferring the Eppendorf tubes to a heating block set at 37°C. After incubation for 90 min, 1-μl aliquots were mixed on a slide with 10 μl of fixative [1% (vol/vol) glutaraldehyde in PME], covered with a coverslip, sealed, and studied in a Bio-Rad MRC600 confocal laser scanning microscope. Microtubules as short as 0.5 μM could be easily distinguished.
For the immunoinhibition assay, MAP60 was mixed with affinity-purified rat anti-65 kDa protein antibodies in PME and incubated for 40 min at room temperature. The rat antiserum was blot affinity-purified against MAP60/62 and has been previously shown to also react with the 68-kDa member of the 65-kDa family, but with no other proteins in the whole cell homogenates (20). Antibody-treated MAP60 at a final concentration of 0.1 μM was subsequently added to rhodamine-tubulin (final concentration, 20 μM) and incubated at 37°C for 2 h. Untreated MAP60 and PME were used as controls.
To test whether MAP60 had a stabilizing effect on microtubules, rhodamine-tubulin was incubated for 60 min in PB containing 0.1 μM MAP60. The microtubules formed in this solution were diluted in PB containing 0.1 μM MAP60. In this way, the concentration of MAP60 was maintained, but the preformed microtubules were diluted 10-, 100-, or 1,000-fold. As a control, rhodamine-tubulin was allowed to self-assemble for 60 min in the absence of MAP60 before dilution in PB. The experiments were performed using 40 μM rhodamine-tubulin to ensure a significant amount of self-assembly in the control. Aliquots were analyzed at different time intervals. By means of pipetting with cut-off tips, shearing forces were kept at a minimum. Repeated pipetting of the same solution of microtubules was found to have no effect on microtubule length distributions.
The cold stability of microtubules assembled with or without MAP60 was determined in a separate series of experiments. Rhodamine-tubulin (25 μM) was incubated for 120 min in the presence of MAP60 (0.1 μM), after which a 1-μl aliquot was taken. The remainder of the solution was put on ice for 45 min before another 1-μl aliquot was analyzed. A solution of 40 μM rhodamine-tubulin assembled for 120 min without MAP60 served as the control.
The length distribution within a microtubule population was determined on prints enlarged on a video screen. Histograms of each sample were constructed on the basis of 400–500 measured microtubules. The data were analyzed by χ2 analysis using minitab software.
Microtubule Bundling Assay.
To detect the presence of a possible microtubule-bundling activity, MAP60 was mixed with taxol-stabilized microtubules. These preformed microtubules were made from either rhodamine-labeled or unlabeled neurotubulin. MAP60 and taxol-stabilized microtubules were mixed at molar ratios of up to 1:2, followed by incubation for 30 min at room temperature. The experiments using rhodamine-labeled microtubules were processed as described above. Unlabeled microtubules were attached to poly-l-lysine-coated slides, fixed with 4% (vol/vol) formaldehyde and 0.1% (vol/vol) glutaraldehyde in PME, and stained with anti-tubulin antibody (Yol 1/34; Amersham) or anti-MAP65 antibody. The secondary antibody was fluorescein isothiocyanate-conjugated rabbit anti-rat (Dako).
Protein Determination.
Protein concentrations were determined by the Bio-Rad protein assay using BSA as a standard. To compensate for the high extinction curve of the BSA, values were corrected by a factor of two per manufacturer’s instructions.
Electron Microscopy.
Microtubules were attached to pyroxylin-carbon coated electron microscopy grids, negative-stained with 2% (vol/vol) uranyl acetate, and examined in a JEOL 1200EX transmission electron microscope.
RESULTS
Isolation of MAP60.
The term “65-kDa MAPs” was given to a group of immunologically related plant MAPs with molecular masses ≈65 kDa (21). In carrot cells, the 65-kDa protein group was found to consist of three proteins with molecular masses of 60, 62, and 68 kDa; antibodies to these carrot proteins crossreacted to the tobacco MAPs (20). The carrot proteins efficiently cosedimented with taxol-stabilized microtubules, even when substantial amounts of unpolymerized tubulin remained in the supernatant (results not shown). This shows that these MAPs have a clear preference for binding to polymeric, as opposed to soluble dimeric, tubulin.
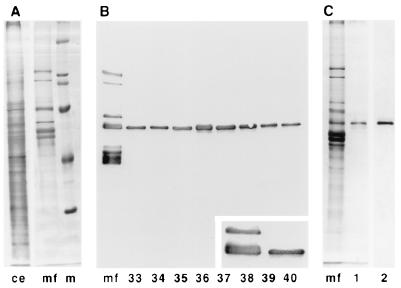
To isolate and further identify the individual proteins, the carrot MAP fraction (Fig. 1A) was fractionated by FPLC using a DEAE 5PW anion exchange column. Individual fractions were blotted onto nitrocellulose paper and screened for the presence of MAPs using whole anti-MAP serum. Fractions that eluted between 140 and 200 mM NaCl were found to be specifically enriched in a 60-kDa protein belonging to the 65-kDa MAP family (Fig. 1B). Fractions with the highest concentrations were pooled and further analyzed. Silver staining showed a single 60-kDa protein band (Fig. 1C). Immunoblotting with affinity-purified rat anti-65 kDa MAP antibodies (Fig. 1C) also confirmed the presence of only one band. The pooled fractions, which contained 35 μg/ml MAP60 (0.6 μM), were used as a source of this MAP in all experiments.
Figure 1.
Purification of MAP60. (A) Protein profiles of carrot cytoskeleton extract (ce) and whole MAP fraction (mf); marker proteins (m) are 205, 116, 97, 68, and 45 kDa. (B) MAPs were fractionated on an FPLC anion exchange column with a linear salt gradient. Individual fractions were analyzed by immunoblotting with whole anti-MAP serum. Fractions 33–40 (146–210 mM salt) were found to contain a protein belonging to the 65-kDa MAPs. The Inset shows this protein to be the 60-kDa MAP. (C) Fractions 36 and 37 (170–186 mM salt) were pooled and further analyzed. Only one band is visible after silver staining on gel (lane 1). Immunoblotting with antibodies against the 65-kDa MAPs confirms the presence of only one protein (lane 2).
Taxol-stabilized microtubules preincubated with MAP60 were recognized in immunofluorescence experiments by anti-65 kDa MAP antibodies, confirming that the FPLC-purified MAP60 still bound to microtubules (results not shown).
Rhodamine Tubulin Assay.
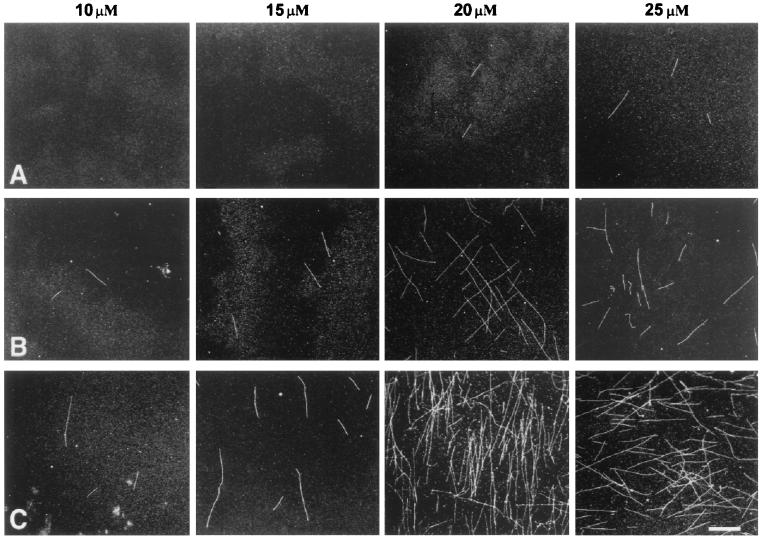
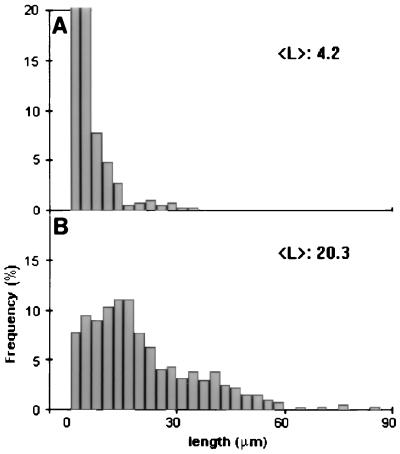
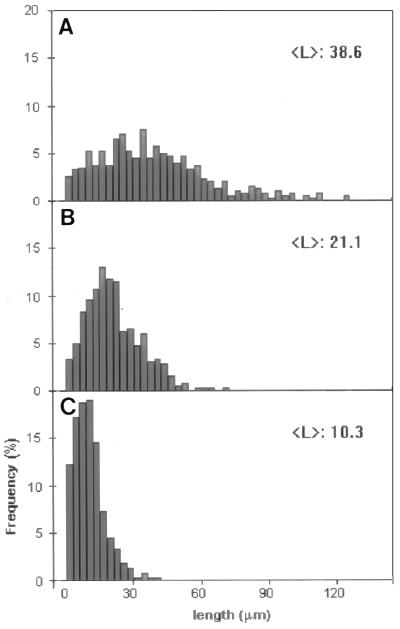
The effects of MAP60 on the polymerization of tubulin were investigated in detail with rhodamine-conjugated brain tubulin. The use of fluorescence-marked tubulin provides a sensitive assay for detecting the formation of microtubules. The tubulin concentrations were chosen such that they were either just below (10 μM and 15 μM) or just above (20 μM and 25 μM) those concentrations that allow self-polymerization (see the controls in Fig. 2). The addition of MAP60 to the non-self-polymerizing concentrations of rhodamine-tubulin induced the formation of morphologically regular microtubules, as was confirmed by electron microscopy (results not shown). The results, however, were moderate: microtubules were never abundant, and a concentration-dependent MAP60 effect, though noticeable, was not pronounced (Fig. 2). On the other hand, when MAP60 was added to concentrations of tubulin that just allowed self-polymerization (i.e., 20 and 25 μM), the effects were far more pronounced: large numbers of microtubules were formed in a MAP60 concentration-dependent fashion (Fig. 2). In a solution containing 25 μM rhodamine-tubulin, there were >10 times as many microtubules per unit area in the presence of 0.2 μM MAP60 than in the control. A significant increase in length was also evident. Whereas microtubules in the control were, on average, 4.2 μm long, this figure rose to 20.2 μm for the MAP microtubules (Fig. 3). Even when formed in the higher concentrations of MAP60, microtubules were typically single rather than bundled.
Figure 2.
The effect of MAP60 on the assembly of rhodamine-tubulin. Rhodamine-tubulin was used at concentrations of 10 μM, 15 μM, 20 μM, and 25 μM. Samples were analyzed after 90 min of incubation. In the controls (no MAP added; A), the first two concentrations did not support self-assembly, whereas a limited degree of self-assembly occurred at concentrations of 20 μM and 25 μM rhodamine-tubulin. Addition of 0.05 μM (B) or 0.2 μM (C) MAP60 to solutions containing 10 or 15 μM rhodamine-tubulin resulted in the assembly of a few microtubules. When, however, MAP60 was mixed with 20 or 25 μM rhodamine-tubulin, the results were more dramatic with the formation of large numbers of microtubules. A concentration-dependent MAP60 effect [compare 0.05 μM (B) and 0.2 μM (C) MAP60], barely detectable at non-self-polymerizing concentrations of rhodamine-tubulin (i.e., 10 or 15 μM), was also much more evident when rhodamine-tubulin was used at self-polymerizing concentrations (i.e., 20 and 25 μM). (Bar = 10 μm.)
Figure 3.
Length distribution of microtubules formed in the absence or presence of MAP60. The analysis was performed on samples from the assembly assay shown in Fig. 2. Controls were: (A) 25 μM rhodamine-tubulin without MAP60 and (B) 25 μM rhodamine-tubulin with 0.2 μM MAP60.
In a variation of these experiments, rhodamine-tubulin at a self-polymerizing concentration that had been preincubated with anti-MAP65 antibodies was added to MAP60. As before, the control experiment without MAP60 showed few, short microtubules, whereas inclusion of MAP60 in the reaction mixture led to a dramatic increase in both number and length of microtubules (results not shown). However, preincubation of MAP60 with the anti-65 kDa antibodies completely abolished this effect; here, as in the control, only few short microtubules were present.
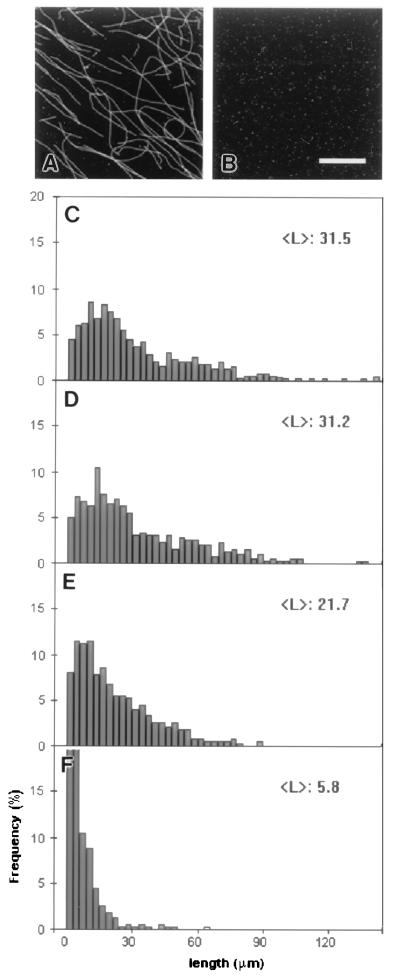
To test the effects of MAP60 on microtubule stabilization, solutions containing 40 μM rhodamine tubulin were allowed to self-polymerize and then diluted, with or without MAP60. Microtubules formed and diluted in the presence of MAP60 were extremely stable. This was evident from the near to identical histograms (P > 0.9) of the length distributions of the microtubules present in the undiluted control at the start of the experiment and in a 10-fold diluted sample after 2 h of incubation (Fig. 4 A, B, and D). In contrast, when microtubules formed in the absence of MAP60 were diluted 10-fold in PB without MAP60, complete depolymerization occurred within 15 min (Fig. 4B). In the presence of MAP60, the first signs of depolymerization became visible when MAP microtubules were diluted 100 times. Depolymerization, however, proceeded slowly, for over a 2 h period, the mean microtubule length only decreased from 31.5 μm to 21.7 μm (Fig. 4E). At a 1000-fold dilution, depolymerization became more prominent, but even here, after 2 h of incubation, there were still some (very short, average length 5.8 μm) microtubules left (Fig. 4F).
Figure 4.
Stability of MAP microtubules after dilution. Rhodamine-tubulin (40 μM) was polymerized for 90 min in the presence of MAP60 (0.1 μM) before dilution in PB supplemented with MAP60 (0.1 μM). The diluted samples were analyzed after 2 h of incubation. (A) Representative image of 10 times diluted MAP microtubules after 2 h of incubation. In the control, microtubules formed and diluted 10-fold without MAP60 completely depolymerize within 15 min (B). The graphs show the length distributions of the microtubules in the undiluted (C) and the 10-fold (D), 100-fold (E), and 1000-fold (F) diluted samples. (Bar = 10 μm.)
Brain microtubules are known to be cold-sensitive and to rapidly depolymerize when incubated at low temperatures, especially when used in combination with calcium (24, 25). In our experiments, no rhodamine-microtubules remained after 10 min on ice (results not shown). Similar results were obtained when microtubules were incubated with BSA (results not shown). However, the inclusion of MAP60 in the microtubule assembly solution altered this sensitivity. Depolymerization was not completely inhibited, but the presence of microtubules after 45 min of incubation on ice or on ice plus calcium demonstrated the enhancement of cold stability (Fig. 5 A–C).
Figure 5.
Graphs showing the effect of MAP60 on the cold and cold/calcium stability of microtubules. Rhodamine-labeled microtubules (40 μM) were assembled in the presence of 0.1 μM MAP60 followed by an incubation on ice for 45 min. (A) Control before cold incubation; (B) microtubules after 45 min on ice; and (C) microtubules after 45 min on ice in the presence of 5 mM CaCl2.
Bundling.
Cyr and Palevitz (15) previously reported that a carrot MAP fraction induced the bundling of microtubules, so it was important to test whether MAP60 might be responsible for this. When tubulin was polymerized in the presence of MAP60, no microtubule bundles were formed (see Fig. 2). However, to test this further, MAP60 was mixed with preformed taxol-stabilized rhodamine-microtubules, thereby increasing the relative concentration of MAP60 (molar ratios of up to 1:2, MAP to tubulin). However, even at these ratios, no differences between the control and MAP60-incubated rhodamine-microtubules were observed. The experiments were repeated in the presence of ATP (1 mM) and 5′-adenylyl imidodiphosphate (10 mM) with the same negative results (results not shown). Similar results were obtained with unlabeled tubulin, showing that the lack of bundling was not due to rhodaminylation of the tubulin.
DISCUSSION
The 65-kDa MAPs are a group of immunologically related proteins that occur in a range of plants, codistributing with all microtubule arrays throughout the cell cycle (20, 21). One of these proteins has now been purified, and we have tested the characteristics of this MAP60 in a series of assays using rhodamine-conjugated neurotubulin.
Used in assembly assays at concentrations of up to 1:50 to tubulin, MAP60 was found to have a profound effect. Tubulin polymerization was significantly stimulated in the presence of MAP60, as shown by the formation of longer microtubules (Figs. 2 and 3), which were more stable than those formed in the tubulin-only control. This latter fact is illustrated by the dilution experiments in which MAP microtubules at 4 μM could be maintained for hours without any sign of depolymerization, whereas MAP-free microtubules diluted to 4 μM depolymerized completely within 15 min. This enhanced stability may also explain the large increase in average microtubule length. Based upon in vitro experiments, it has been postulated that animal MAPs stimulate the growth of microtubules not by increasing the rate of polymerization but by decreasing disassembly (7, 26, 27). The dilution experiments showed that disassembly of microtubules was markedly slower in the presence of MAP60, which may therefore stimulate microtubule growth in the same way neuronal MAPs do, that is, by decreasing disassembly.
It is noteworthy that for MAP60 to have a strong effect, the tubulin concentration had to be raised above the threshold value required for tubulin assembly in the control (see Fig. 2). This suggests that MAP60 did not influence, or only marginally influenced, the critical concentration for tubulin assembly. It is, however, significant that, under these conditions, MAP60 clearly increased the number of microtubules above those seen in the controls. This indicates that the plant MAP promotes the formation of microtubule starts, even though it may not have a dramatic effect in lowering the critical concentration for assembly. Similar properties are displayed by MAP1b of brain, which promotes microtubule nucleation and growth efficiently despite its small impact on the critical concentration (7).
Perhaps the most notable effect of MAP60 was its ability to enhance the stability of microtubules to cold or to cold/calcium. Subpopulations of cold-stable microtubules have been found in animal cells (28, 29) and are thought to be the result of nontubulin factors (30). Induction of cold stability is a characteristic found only in a handful of animal MAPs of which STOP (stable tubulin-only protein) is perhaps the best known (31). Like MAP60, STOP stabilizes microtubules against depolymerization induced by dilution, exerts its effect at relatively low molar ratios to tubulin, and codistributes with microtubules within the cell (32, 33).
The possession of cold-stable microtubules is of particular importance to plants in which cytoskeletal assemblies must function at low ambient temperatures. In plants, the induction of cold stability has been correlated with the expression of different β-tubulin genes (34), although nontubulin factors have also been implicated (35). Bokros et al. (36) have presented evidence that the C terminus of β-tubulin may be the regulatory domain involved in the sensitivity to depolymerization by cold and calcium. Removal of a small part of the C terminus markedly improved the resistance of tubulin to cold depolymerization. Enhanced cold stability may also be the result of MAPs binding to this regulatory domain. In animals, the C termini of both α- and β-tubulin are regulatory domains, and binding of MAPs has been shown to promote the polymerization of tubulin and stabilize microtubules (5, 37, 38). It is therefore conceivable that MAP60 promotes the assembly of neurotubulin by binding to a regulatory domain of neurotubulin, and, being from a plant source, performs the same function in plant cells.
There have been previous hints that plants possess factors that stabilize microtubules. Sonobe and Takahashi (39) for instance, found that microtubules associated with plasma membrane ghosts were cold-stable, although an extract of evacuolated tobacco cells increased the cold sensitivity. Cyr and Palevitz (15) obtained a MAP fraction from carrot cells that induced bundling of neurotubules, which were then cold-stable. MAP60, being also derived from carrots, could be responsible for the cold stability we observed. However, purified MAP60 does not induce bundling, and it is likely that other factors are responsible for this.
Only one other plant cytoskeletal protein has been demonstrated to influence microtubule stability. Mizuno (40) has described a basic, fibrous, 50-kDa protein that has similarities to intermediate filament proteins. However, this protein is unlike MAP60 in that it can self-assemble into filaments, and it also does not codistribute with cortical microtubules and induce cold stability. MAP60 would therefore appear to be the first plant MAP to induce cold stability.
To extract these MAPs, carrot cytoskeletons were vigorously shaken for 60 min on ice in cold/calcium; hence, the presence of MAP60 in cytoskeletons does not provide absolute protection against cold. That the effect of MAP60 is relative is illustrated by the fact that although it does enhance the resistance of neurotubules to the cold in vitro, it does not completely prevent depolymerization, and the degree of protection is even less when calcium is added (see Fig. 5).
The ability of MAP60 to stabilize microtubules in vitro most certainly has implications for the role of this MAP in vivo. Extrapolating from the results on neurotubulin, MAP60 is likely to have a cold-stabilizing effect on microtubules in plant cells. Plant microtubules are known to be highly dynamic (13) and able to switch between transverse and longitudinal arrays in 20–40 min (14) upon stimulation. The roles microtubules play in cellular processes depend on the balance between stability and instability. Stability allows microtubules to act as a quasirigid framework, whereas instability allows adaptability. The reorientation of microtubules in living plant cells seems to involve the formation of new microtubules, whereas old microtubules are depolymerized (14, 41). Selective stabilization of microtubules has been discussed as being an important mechanism in morphogenesis (42), and in view of our results with MAP60, it is tempting to speculate that it may help to control microtubule turnover in vivo.
Acknowledgments
We thank Dr. C. Wymer and Dr. G. Calder for helpful discussions and critical reading of the manuscript. C.W.L. and J.C. were funded by the John Innes Centre by way of a grant-in-aid by the Biotechnology and Biological Sciences Research Council. T.R. was funded by an European Community Human Capital Mobility Fellowship.
ABBREVIATIONS
- MAP
microtubule-associated protein
- FPLC
fast protein liquid chromotography
References
- 1.Murphy D B, Borisy G G. Proc Natl Acad Sci USA. 1975;72:2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy D B, Johnson K A, Borisy G G. J Mol Biol. 1977;117:33–52. doi: 10.1016/0022-2836(77)90021-3. [DOI] [PubMed] [Google Scholar]
- 3.Weingarten M D, Lockwood A H, Hwo S, Kirschner M W. Proc Natl Acad Sci USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olmsted J B. Annu Rev Cell Biol. 1986;2:421–457. doi: 10.1146/annurev.cb.02.110186.002225. [DOI] [PubMed] [Google Scholar]
- 5.Maccioni R B, Cambiazo V. Phys Rev. 1995;75:835–864. doi: 10.1152/physrev.1995.75.4.835. [DOI] [PubMed] [Google Scholar]
- 6.Mandelkow E, Mandelkow E-M. Curr Opin Cell Biol. 1995;7:72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 7.Vandecandelaere A, Pedrotti B, Utton M A, Calvert R A, Bayley P M. Cell Motil Cytoskeleton. 1996;35:134–146. doi: 10.1002/(SICI)1097-0169(1996)35:2<134::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 8.Bré M H, Karsenti E. Cell Motil Cytoskeleton. 1990;15:88–98. doi: 10.1002/cm.970150205. [DOI] [PubMed] [Google Scholar]
- 9.Pryer N K, Walker R A, Skeen V P, Bourns B D, Soboeiro M F, Salmon E D. J Cell Sci. 1992;103:965–976. doi: 10.1242/jcs.103.4.965. [DOI] [PubMed] [Google Scholar]
- 10.Chapin S J, Bulinsky J C, Gundersen G G. Nature (London) 1991;349:24. doi: 10.1038/349024a0. [DOI] [PubMed] [Google Scholar]
- 11.Gustke N, Trinczek B, Biernat J, Mandelkow E-M, Mandelkow E. Biochemistry. 1994;33:9511–9522. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- 12.Lee G, Brandt R. Trends Cell Biol. 1992;2:286–289. doi: 10.1016/0962-8924(92)90106-w. [DOI] [PubMed] [Google Scholar]
- 13.Hush J M, Wadsworth P, Callahan D A, Hepler P K. J Cell Sci. 1994;107:775–784. doi: 10.1242/jcs.107.4.775. [DOI] [PubMed] [Google Scholar]
- 14.Yuan M, Shaw P J, Warn R M, Lloyd C W. Proc Natl Acad Sci USA. 1994;91:6050–6053. doi: 10.1073/pnas.91.13.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cyr R J, Palevitz B A. Planta. 1989;177:245–260. doi: 10.1007/BF00392813. [DOI] [PubMed] [Google Scholar]
- 16.Maekawa T, Ogihara S, Murofushi H, Nagai R. Protoplasma. 1990;158:10–18. [Google Scholar]
- 17.Schellenbaum P, Vantard M, Peter C, Fellous A, Lambert A-M. Plant J. 1993;3:253–260. [Google Scholar]
- 18.Shibaoka H, Asada T, Yamamoto S, Sonobe S. J Microsc (Oxford) 1995;181:145–152. [Google Scholar]
- 19.Vantard M, Schellenbaum P, Fellous A, Lambert A M. Biochemistry. 1991;30:9334–9340. doi: 10.1021/bi00102a028. [DOI] [PubMed] [Google Scholar]
- 20.Chan J, Rutten T, Lloyd C W. Plant J. 1996;10:251–259. doi: 10.1046/j.1365-313x.1996.10020251.x. [DOI] [PubMed] [Google Scholar]
- 21.Jiang C-J, Sonobe S. J Cell Sci. 1993;105:891–901. doi: 10.1242/jcs.105.4.891. [DOI] [PubMed] [Google Scholar]
- 22.Wymer, C. L., Shaw, P. J., Warn, R. M. & Lloyd, C. W. (1997) Plant J., in press.
- 23.Hyman A, Drechsel D, Kellogg D, Salser S, Sawin P, Steffen P, Wordeman L, Mitchison T. Methods Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- 24.Mandelkow E, Mandelkow E-M. J Mol Biol. 1985;181:123–135. doi: 10.1016/0022-2836(85)90330-4. [DOI] [PubMed] [Google Scholar]
- 25.Solomon F. Biochemistry. 1977;16:358–363. doi: 10.1021/bi00622a003. [DOI] [PubMed] [Google Scholar]
- 26.Cassimeris L. Cell Motil Cytoskeleton. 1993;26:275–281. doi: 10.1002/cm.970260402. [DOI] [PubMed] [Google Scholar]
- 27.Dhamodharan R, Wadsworth P. J Cell Sci. 1995;108:1679–1689. doi: 10.1242/jcs.108.4.1679. [DOI] [PubMed] [Google Scholar]
- 28.Brinkley B R, Cartwright J. Ann NY Acad Sci. 1975;253:428–439. doi: 10.1111/j.1749-6632.1975.tb19218.x. [DOI] [PubMed] [Google Scholar]
- 29.Salmon E D, Begg D A. J Cell Biol. 1980;85:853–865. doi: 10.1083/jcb.85.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones D H, Grey E, Barron J. J Neurocytol. 1980;9:493–504. doi: 10.1007/BF01204838. [DOI] [PubMed] [Google Scholar]
- 31.Job D, Margolis R L. Biochemistry. 1984;23:3025–3031. doi: 10.1021/bi00308a028. [DOI] [PubMed] [Google Scholar]
- 32.Margolis R L, Job D. In: Microtubules. Hyams J S, Lloyd C W, editors. New York: Wiley–Liss; 1994. pp. 221–228. [Google Scholar]
- 33.Margolis R L, Rauch C T, Pirollet F, Job D. EMBO J. 1990;9:4099–4012. doi: 10.1002/j.1460-2075.1990.tb07631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu B, Snustad D P, Carter J V. Plant Physiol. 1993;103:371–377. doi: 10.1104/pp.103.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizuno K. Plant Physiol. 1992;100:740–748. doi: 10.1104/pp.100.2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bokros C L, Hughdahl J D, Blumenthal S S D, Morejohn L C. Plant Cell Environ. 1996;19:539–548. [Google Scholar]
- 37.Sackett D L, Bhattaharyya B, Wolff J. J Biol Chem. 1985;260:43–45. [PubMed] [Google Scholar]
- 38.Wiche G, Oberkannis C, Himmler A. Int Rev Cytol. 1991;124:217–273. doi: 10.1016/s0074-7696(08)61528-4. [DOI] [PubMed] [Google Scholar]
- 39.Sonobe S, Takahashi S. Plant Cell Physiol. 1994;35:451–460. [Google Scholar]
- 40.Mizuno K. Protoplasma. 1995;186:99–112. [Google Scholar]
- 41.Lloyd C W, Shaw P J, Warn R M, Yuan M. J Microsc (Oxford) 1996;181:140–144. [Google Scholar]
- 42.Kirschner M, Mitchison T. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]