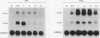Abstract
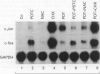
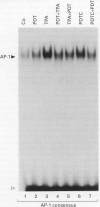
Photodynamic therapy (PDT) is currently under investigation in phase II and III clinical studies for the treatment of tumours in superficial localisations. Thus far, the underlying mechanisms of PDT regarding cellular responses and gene regulation are poorly understood. Photochemically generated singlet oxygen (1O2) is mainly responsible for cytotoxicity induced by PDT. If targeted cells are not disintegrated, photo-oxidative stress leads to transcription and translation of various stress response and cytokine genes. Tumour necrosis factor (TNF) alpha, interleukin (IL) 1 and IL-6 are strongly induced by photodynamic treatment, supporting inflammatory action and immunological anti-tumour responses. To investigate the first steps of gene activation, this study focused on the proto-oncogenes c-jun and c-fos, both coding for the transcription factor activator protein 1 (AP-1), which was found to mediate IL-6 gene expression. We here determine the effects of photodynamic treatment on transcriptional regulation and DNA binding of transcription factor AP-1 in order to understand the modulation of subsequent regulatory steps. Photodynamic treatment of epithelial HeLa cells was performed by incubation with Photofrin and illumination with 630 nm laser light in vitro. Expression of the c-jun and c-fos genes was determined by way of Northern blot analysis, and DNA-binding activity of the transcription factor AP-1 was evaluated by electrophoretic mobility shift assay (EMSA). Photofrin-mediated photosensitisation of HeLa cells resulted in a rapid and dose-dependent induction of both genes but preferential expression of c-jun. Compared with the transient expression of c-jun and c-fos by phorbol ester stimulation, photodynamic treatment led to a prolonged activation pattern of both immediate early genes. Furthermore, mRNA stability studies revealed an increased half-life of c-jun and c-fos transcripts resulting from photosensitisation. Although mRNA accumulation after PDT was stronger and more prolonged compared with phorbol ester stimulation, with regard to AP-1 DNA-binding activity, phorbol ester was more efficient. Surprisingly, in addition to the activation of AP-1 DNA-binding via PDT, photodynamic treatment can decrease AP-1 DNA-binding of other strong inducers, such as the protein kinase C-mediated pathway of phorbol esters and the antioxidant pyrrolidine dithiocarbamate (PDTC). This study demonstrates a strong induction of c-jun and c-fos expression by PDT, with prolonged kinetics and mRNA stabilisation as compared with activation by phorbol esters. Interestingly, this observation is not coincident with an overinduction of AP-1 DNA-binding, hence suggesting that post-translational modifications are dominant regulatory mechanisms after PDT that tightly control AP-1 activity in the nucleus thus limiting the risk of deregulated oncogene expression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Amstad P. A., Krupitza G., Cerutti P. A. Mechanism of c-fos induction by active oxygen. Cancer Res. 1992 Jul 15;52(14):3952–3960. [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Hoey B. M., Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6(6):593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Bannister A. J., Cook A., Kouzarides T. In vitro DNA binding activity of Fos/Jun and BZLF1 but not C/EBP is affected by redox changes. Oncogene. 1991 Jul;6(7):1243–1250. [PubMed] [Google Scholar]
- Bos T. J., Monteclaro F. S., Mitsunobu F., Ball A. R., Jr, Chang C. H., Nishimura T., Vogt P. K. Efficient transformation of chicken embryo fibroblasts by c-Jun requires structural modification in coding and noncoding sequences. Genes Dev. 1990 Oct;4(10):1677–1687. doi: 10.1101/gad.4.10.1677. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Burgunder J. M., Varriale A., Lauterburg B. H. Effect of N-acetylcysteine on plasma cysteine and glutathione following paracetamol administration. Eur J Clin Pharmacol. 1989;36(2):127–131. doi: 10.1007/BF00609183. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Shyu A. B. Selective degradation of early-response-gene mRNAs: functional analyses of sequence features of the AU-rich elements. Mol Cell Biol. 1994 Dec;14(12):8471–8482. doi: 10.1128/mcb.14.12.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., You Y., Shyu A. B. Two cellular proteins bind specifically to a purine-rich sequence necessary for the destabilization function of a c-fos protein-coding region determinant of mRNA instability. Mol Cell Biol. 1992 Dec;12(12):5748–5757. doi: 10.1128/mcb.12.12.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Lau L. F., Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991 May;11(5):2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dérijard B., Hibi M., Wu I. H., Barrett T., Su B., Deng T., Karin M., Davis R. J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994 Mar 25;76(6):1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Evans S., Matthews W., Perry R., Fraker D., Norton J., Pass H. I. Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. J Natl Cancer Inst. 1990 Jan 3;82(1):34–39. doi: 10.1093/jnci/82.1.34. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frame M. C., Wilkie N. M., Darling A. J., Chudleigh A., Pintzas A., Lang J. C., Gillespie D. A. Regulation of AP-1/DNA complex formation in vitro. Oncogene. 1991 Feb;6(2):205–209. [PubMed] [Google Scholar]
- Gentz R., Rauscher F. J., 3rd, Abate C., Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989 Mar 31;243(4899):1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Luna M., Ferrario A., Rucker N. Increased transcription and translation of heme oxygenase in Chinese hamster fibroblasts following photodynamic stress or Photofrin II incubation. Photochem Photobiol. 1991 Feb;53(2):275–279. doi: 10.1111/j.1751-1097.1991.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Hai T., Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori K., Angel P., Le Beau M. M., Karin M. Structure and chromosomal localization of the functional intronless human JUN protooncogene. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9148–9152. doi: 10.1073/pnas.85.23.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook N. J., Fornace A. J., Jr Response to adversity: molecular control of gene activation following genotoxic stress. New Biol. 1991 Sep;3(9):825–833. [PubMed] [Google Scholar]
- Hsu W., Kerppola T. K., Chen P. L., Curran T., Chen-Kiang S. Fos and Jun repress transcription activation by NF-IL6 through association at the basic zipper region. Mol Cell Biol. 1994 Jan;14(1):268–276. doi: 10.1128/mcb.14.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Ivashkiv L. B., Liou H. C., Kara C. J., Lamph W. W., Verma I. M., Glimcher L. H. mXBP/CRE-BP2 and c-Jun form a complex which binds to the cyclic AMP, but not to the 12-O-tetradecanoylphorbol-13-acetate, response element. Mol Cell Biol. 1990 Apr;10(4):1609–1621. doi: 10.1128/mcb.10.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabnick K. S., Housman D. E. Determinants that contribute to cytoplasmic stability of human c-fos and beta-globin mRNAs are located at several sites in each mRNA. Mol Cell Biol. 1988 Aug;8(8):3244–3250. doi: 10.1128/mcb.8.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallunki T., Su B., Tsigelny I., Sluss H. K., Dérijard B., Moore G., Davis R., Karin M. JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev. 1994 Dec 15;8(24):2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- Kataoka K., Igarashi K., Itoh K., Fujiwara K. T., Noda M., Yamamoto M., Nishizawa M. Small Maf proteins heterodimerize with Fos and may act as competitive repressors of the NF-E2 transcription factor. Mol Cell Biol. 1995 Apr;15(4):2180–2190. doi: 10.1128/mcb.15.4.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Selective DNA bending by a variety of bZIP proteins. Mol Cell Biol. 1993 Sep;13(9):5479–5489. doi: 10.1128/mcb.13.9.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kick G., Messer G., Goetz A., Plewig G., Kind P. Photodynamic therapy induces expression of interleukin 6 by activation of AP-1 but not NF-kappa B DNA binding. Cancer Res. 1995 Jun 1;55(11):2373–2379. [PubMed] [Google Scholar]
- Kim R., Beck W. T. Differences between drug-sensitive and -resistant human leukemic CEM cells in c-jun expression, AP-1 DNA-binding activity, and formation of Jun/Fos family dimers, and their association with internucleosomal DNA ladders after treatment with VM-26. Cancer Res. 1994 Sep 15;54(18):4958–4966. [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988 Dec 15;336(6200):646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., Banerjee P., Nikolakaki E., Dai T., Rubie E. A., Ahmad M. F., Avruch J., Woodgett J. R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994 May 12;369(6476):156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lee W. M., Lin C., Curran T. Activation of the transforming potential of the human fos proto-oncogene requires message stabilization and results in increased amounts of partially modified fos protein. Mol Cell Biol. 1988 Dec;8(12):5521–5527. doi: 10.1128/mcb.8.12.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunig A., Staub F., Peters J., Heimann A., Csapo C., Kempski O., Goetz A. E. Relation of early Photofrin uptake to photodynamically induced phototoxicity and changes of cell volume in different cell lines. Eur J Cancer. 1994;30A(1):78–83. doi: 10.1016/s0959-8049(05)80023-1. [DOI] [PubMed] [Google Scholar]
- Li Y., Jaiswal A. K. Human antioxidant-response-element-mediated regulation of type 1 NAD(P)H:quinone oxidoreductase gene expression. Effect of sulfhydryl modifying agents. Eur J Biochem. 1994 Nov 15;226(1):31–39. doi: 10.1111/j.1432-1033.1994.tb20023.x. [DOI] [PubMed] [Google Scholar]
- Luna M. C., Wong S., Gomer C. J. Photodynamic therapy mediated induction of early response genes. Cancer Res. 1994 Mar 1;54(5):1374–1380. [PubMed] [Google Scholar]
- Manome Y., Datta R., Taneja N., Shafman T., Bump E., Hass R., Weichselbaum R., Kufe D. Coinduction of c-jun gene expression and internucleosomal DNA fragmentation by ionizing radiation. Biochemistry. 1993 Oct 12;32(40):10607–10613. doi: 10.1021/bi00091a010. [DOI] [PubMed] [Google Scholar]
- Meijlink F., Curran T., Miller A. D., Verma I. M. Removal of a 67-base-pair sequence in the noncoding region of protooncogene fos converts it to a transforming gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4987–4991. doi: 10.1073/pnas.82.15.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Schreck R., Baeuerle P. A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993 May;12(5):2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Nose K., Shibanuma M., Kikuchi K., Kageyama H., Sakiyama S., Kuroki T. Transcriptional activation of early-response genes by hydrogen peroxide in a mouse osteoblastic cell line. Eur J Biochem. 1991 Oct 1;201(1):99–106. doi: 10.1111/j.1432-1033.1991.tb16261.x. [DOI] [PubMed] [Google Scholar]
- Papavassiliou A. G., Bohmann K., Bohmann D. Determining the effect of inducible protein phosphorylation on the DNA-binding activity of transcription factors. Anal Biochem. 1992 Jun;203(2):302–309. doi: 10.1016/0003-2697(92)90317-z. [DOI] [PubMed] [Google Scholar]
- Pass H. I. Photodynamic therapy in oncology: mechanisms and clinical use. J Natl Cancer Inst. 1993 Mar 17;85(6):443–456. doi: 10.1093/jnci/85.6.443. [DOI] [PubMed] [Google Scholar]
- Plet A., Eick D., Blanchard J. M. Elongation and premature termination of transcripts initiated from c-fos and c-myc promoters show dissimilar patterns. Oncogene. 1995 Jan 19;10(2):319–328. [PubMed] [Google Scholar]
- Rahmsdorf H. J., Schönthal A., Angel P., Litfin M., Rüther U., Herrlich P. Posttranscriptional regulation of c-fos mRNA expression. Nucleic Acids Res. 1987 Feb 25;15(4):1643–1659. doi: 10.1093/nar/15.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lanahan A., Perez-Albuerne E., Nathans D. jun-D: a third member of the jun gene family. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1500–1503. doi: 10.1073/pnas.86.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Komitowski D., Schubert F. R., Wagner E. F. c-fos expression induces bone tumors in transgenic mice. Oncogene. 1989 Jul;4(7):861–865. [PubMed] [Google Scholar]
- Saez E., Rutberg S. E., Mueller E., Oppenheim H., Smoluk J., Yuspa S. H., Spiegelman B. M. c-fos is required for malignant progression of skin tumors. Cell. 1995 Sep 8;82(5):721–732. doi: 10.1016/0092-8674(95)90469-7. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Ransone L. J., Lamph W. W., Verma I. M. Direct interaction between fos and jun nuclear oncoproteins: role of the 'leucine zipper' domain. Nature. 1988 Dec 15;336(6200):692–695. doi: 10.1038/336692a0. [DOI] [PubMed] [Google Scholar]
- Scanlon K. J., Ishida H., Kashani-Sabet M. Ribozyme-mediated reversal of the multidrug-resistant phenotype. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11123–11127. doi: 10.1073/pnas.91.23.11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk H., Klein M., Erdbrügger W., Dröge W., Schulze-Osthoff K. Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-kappa B and AP-1. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1672–1676. doi: 10.1073/pnas.91.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck R., Meier B., Männel D. N., Dröge W., Baeuerle P. A. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992 May 1;175(5):1181–1194. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu A. B., Greenberg M. E., Belasco J. G. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989 Jan;3(1):60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- Smeyne R. J., Vendrell M., Hayward M., Baker S. J., Miao G. G., Schilling K., Robertson L. M., Curran T., Morgan J. I. Continuous c-fos expression precedes programmed cell death in vivo. Nature. 1993 May 13;363(6425):166–169. doi: 10.1038/363166a0. [DOI] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G., Wang F., Pan Y. C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg S., Rahmsdorf H. J., Herrlich P., Kaina B. Overexpression of c-fos increases recombination frequency in human osteosarcoma cells. Carcinogenesis. 1993 May;14(5):925–928. doi: 10.1093/carcin/14.5.925. [DOI] [PubMed] [Google Scholar]