Abstract
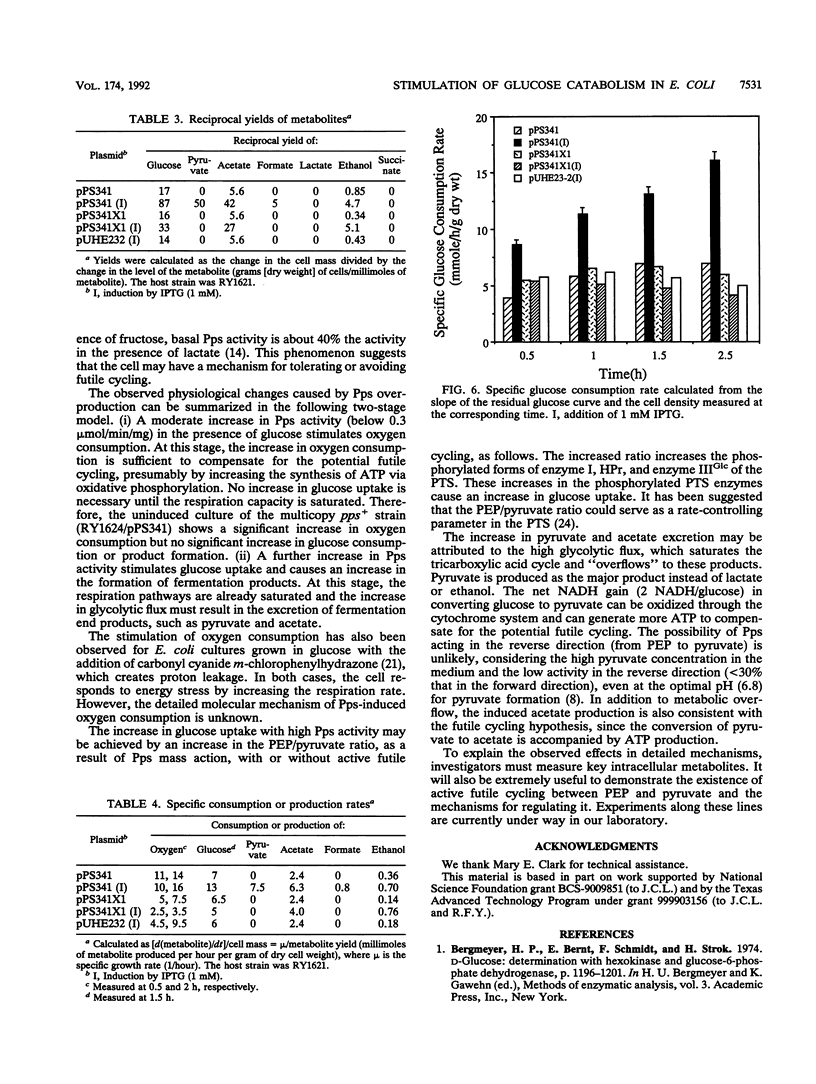
Fifteen-fold overexpression of phosphoenolpyruvate synthase (Pps) (EC 2.7.9.2) in Escherichia coli stimulated oxygen consumption in glucose minimal medium. A further increase in Pps overexpression to 30-fold stimulated glucose consumption by approximately 2-fold and resulted in an increased excretion of pyruvate and acetate. Insertion of two codons at the PvuII site in the pps gene abolished the enzymatic activity and eliminated the above-described effects. Both the active and the inactive proteins were detected at the predicted molecular weight by polyacrylamide gel electrophoresis. Therefore, the observed physiological changes were due to the activity of Pps. The higher specific rates of consumption of oxygen and glucose indicate a potential futile cycle between phosphoenolpyruvate (PEP) and pyruvate. A model for the stimulation of glucose uptake is presented; it involves an increased PEP/pyruvate ratio caused by the overexpressed Pps activity, leading to a stimulation of the PEP:sugar phosphotransferase system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambost J. P., Fraenkel D. G. The use of 6-labeled glucose to assess futile cycling in Escherichia coli. J Biol Chem. 1980 Apr 10;255(7):2867–2869. [PubMed] [Google Scholar]
- Chen W. J., Gross L., Joho K. E., McAllister W. T. A modified kanamycin-resistance cassette to facilitate two-codon insertion mutagenesis. Gene. 1992 Feb 1;111(1):143–144. doi: 10.1016/0378-1119(92)90617-x. [DOI] [PubMed] [Google Scholar]
- Chin A. M., Feldheim D. A., Saier M. H., Jr Altered transcriptional patterns affecting several metabolic pathways in strains of Salmonella typhimurium which overexpress the fructose regulon. J Bacteriol. 1989 May;171(5):2424–2434. doi: 10.1128/jb.171.5.2424-2434.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A. M., Feucht B. U., Saier M. H., Jr Evidence for regulation of gluconeogenesis by the fructose phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1987 Feb;169(2):897–899. doi: 10.1128/jb.169.2.897-899.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. The direct synthesis of phosphoenolpyruvate from pyruvate by Escherichia coli. Proc R Soc Lond B Biol Sci. 1967 Sep 12;168(1012):263–280. doi: 10.1098/rspb.1967.0065. [DOI] [PubMed] [Google Scholar]
- Daldal F., Fraenkel D. G. Assessment of a futile cycle involving reconversion of fructose 6-phosphate to fructose 1,6-bisphosphate during gluconeogenic growth of Escherichia coli. J Bacteriol. 1983 Jan;153(1):390–394. doi: 10.1128/jb.153.1.390-394.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerse R. H., Izzo F., Postma P. W. The PEP: fructose phosphotransferase system in Salmonella typhimurium: FPr combines enzyme IIIFru and pseudo-HPr activities. Mol Gen Genet. 1989 Apr;216(2-3):517–525. doi: 10.1007/BF00334399. [DOI] [PubMed] [Google Scholar]
- Geerse R. H., Ruig C. R., Schuitema A. R., Postma P. W. Relationship between pseudo-HPr and the PEP: fructose phosphotransferase system in Salmonella typhimurium and Escherichia coli. Mol Gen Genet. 1986 Jun;203(3):435–444. doi: 10.1007/BF00422068. [DOI] [PubMed] [Google Scholar]
- Geerse R. H., van der Pluijm J., Postma P. W. The repressor of the PEP:fructose phosphotransferase system is required for the transcription of the pps gene of Escherichia coli. Mol Gen Genet. 1989 Aug;218(2):348–352. doi: 10.1007/BF00331288. [DOI] [PubMed] [Google Scholar]
- Goldie A. H., Sanwal B. D. Genetic and physiological characterization of Escherichia coli mutants deficient in phosphoenolpyruvate carboxykinase activity. J Bacteriol. 1980 Mar;141(3):1115–1121. doi: 10.1128/jb.141.3.1115-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A., Casadaban M. J. Mini-mu bacteriophage with plasmid replicons for in vivo cloning and lac gene fusing. J Bacteriol. 1986 Oct;168(1):357–364. doi: 10.1128/jb.168.1.357-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Wittekind M., Nomura M., Shiba K., Yura T., Miura A., Nashimoto H. A temperature-sensitive mutant of E. coli exhibiting slow processing of exported proteins. Cell. 1983 Mar;32(3):789–797. doi: 10.1016/0092-8674(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Kornberg H. The roles of HPr and FPr in the utilization of fructose by Escherichia coli. FEBS Lett. 1986 Jan 1;194(1):12–15. doi: 10.1016/0014-5793(86)80042-4. [DOI] [PubMed] [Google Scholar]
- Nakano S., Onoda T. Effect of protonophore on growth of Escherichia coli. J Basic Microbiol. 1989;29(3):163–169. doi: 10.1002/jobm.3620290310. [DOI] [PubMed] [Google Scholar]
- Narindrasorasak S., Bridger W. A. Phosphoenolypyruvate synthetase of Escherichia coli: molecular weight, subunit composition, and identification of phosphohistidine in phosphoenzyme intermediate. J Biol Chem. 1977 May 25;252(10):3121–3127. [PubMed] [Google Scholar]
- Niersbach M., Kreuzaler F., Geerse R. H., Postma P. W., Hirsch H. J. Cloning and nucleotide sequence of the Escherichia coli K-12 ppsA gene, encoding PEP synthase. Mol Gen Genet. 1992 Jan;231(2):332–336. doi: 10.1007/BF00279808. [DOI] [PubMed] [Google Scholar]