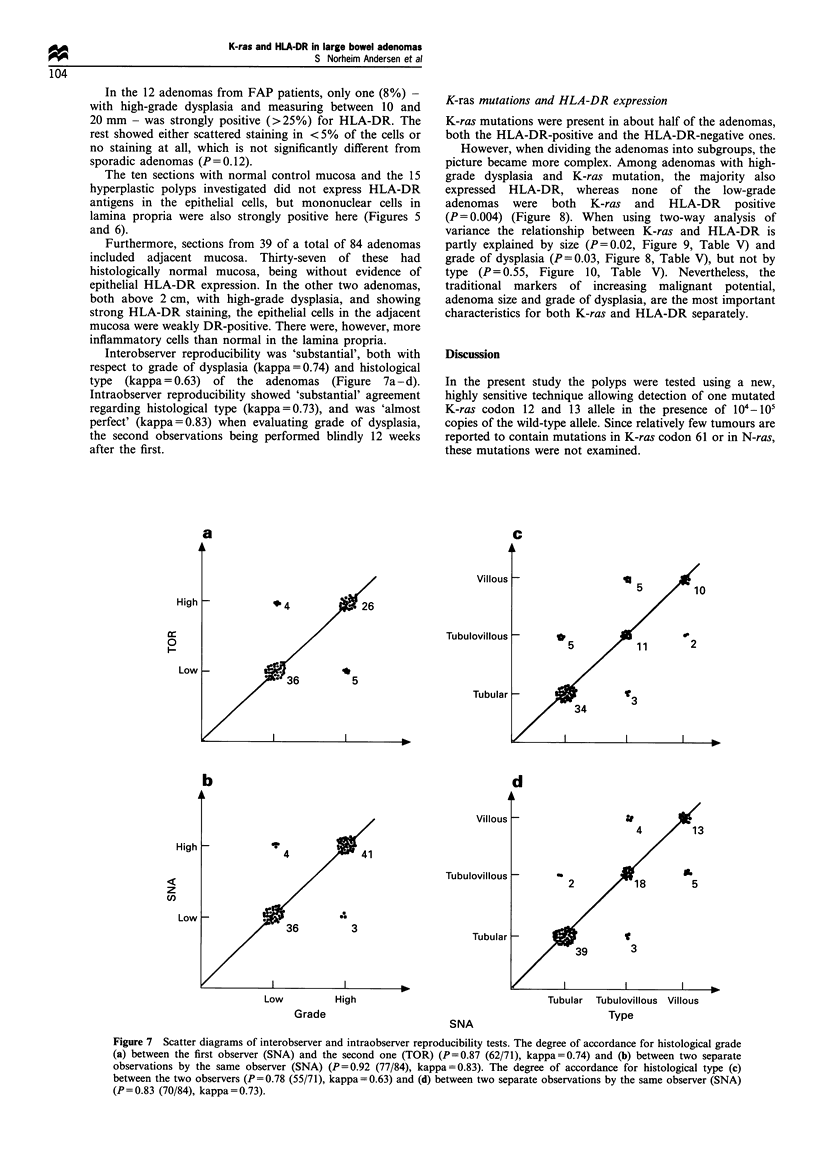
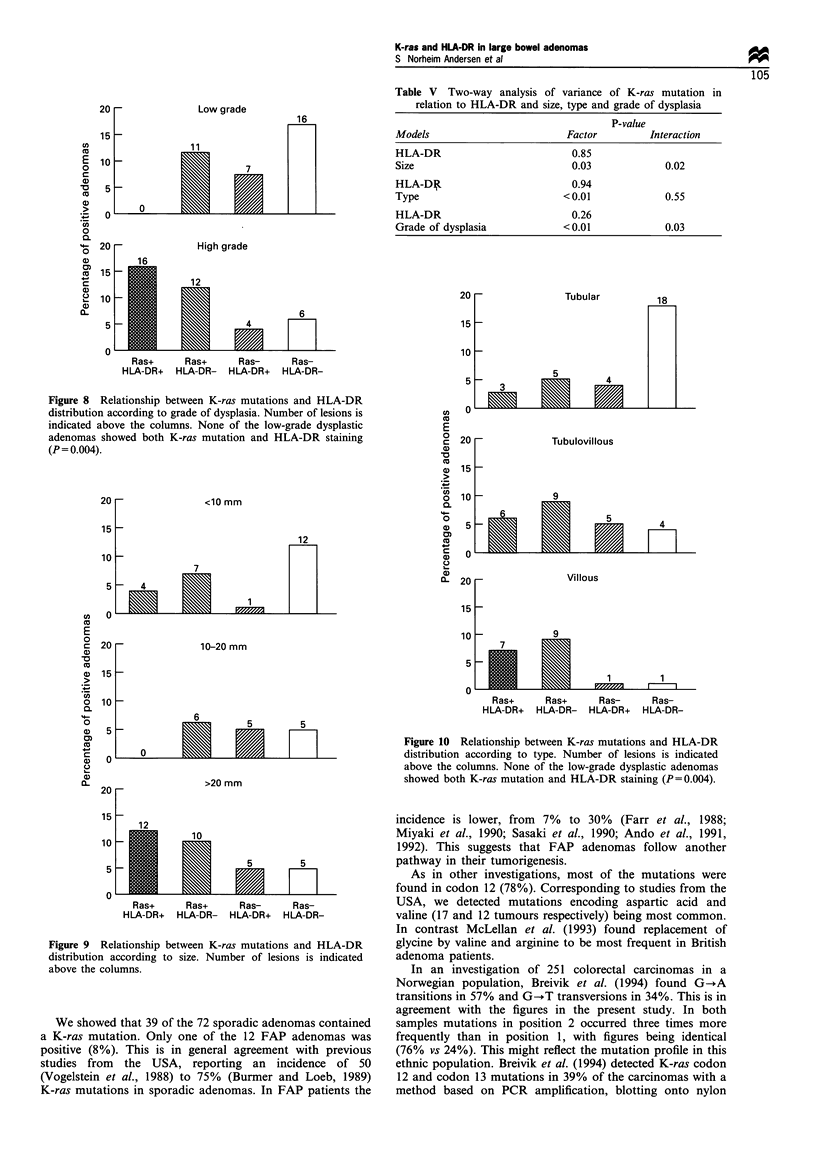
Abstract
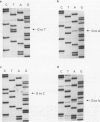
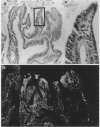
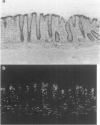
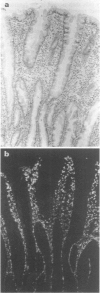
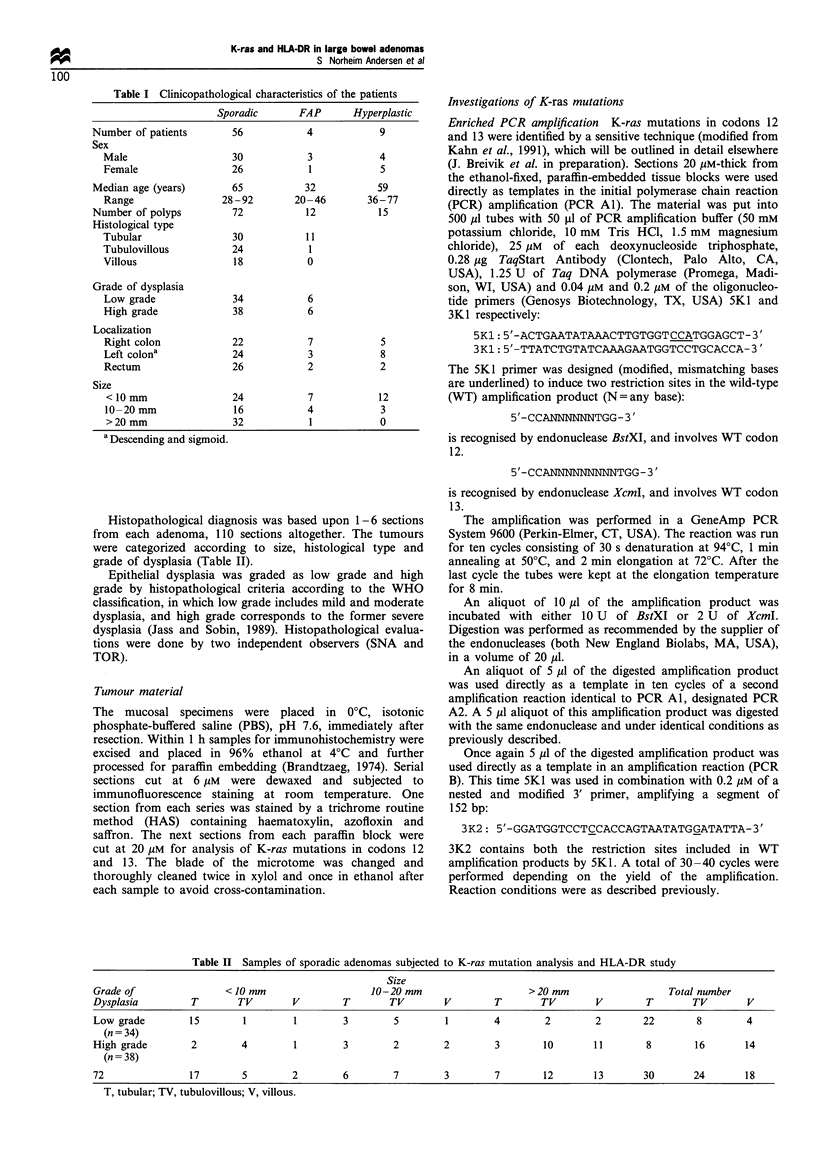
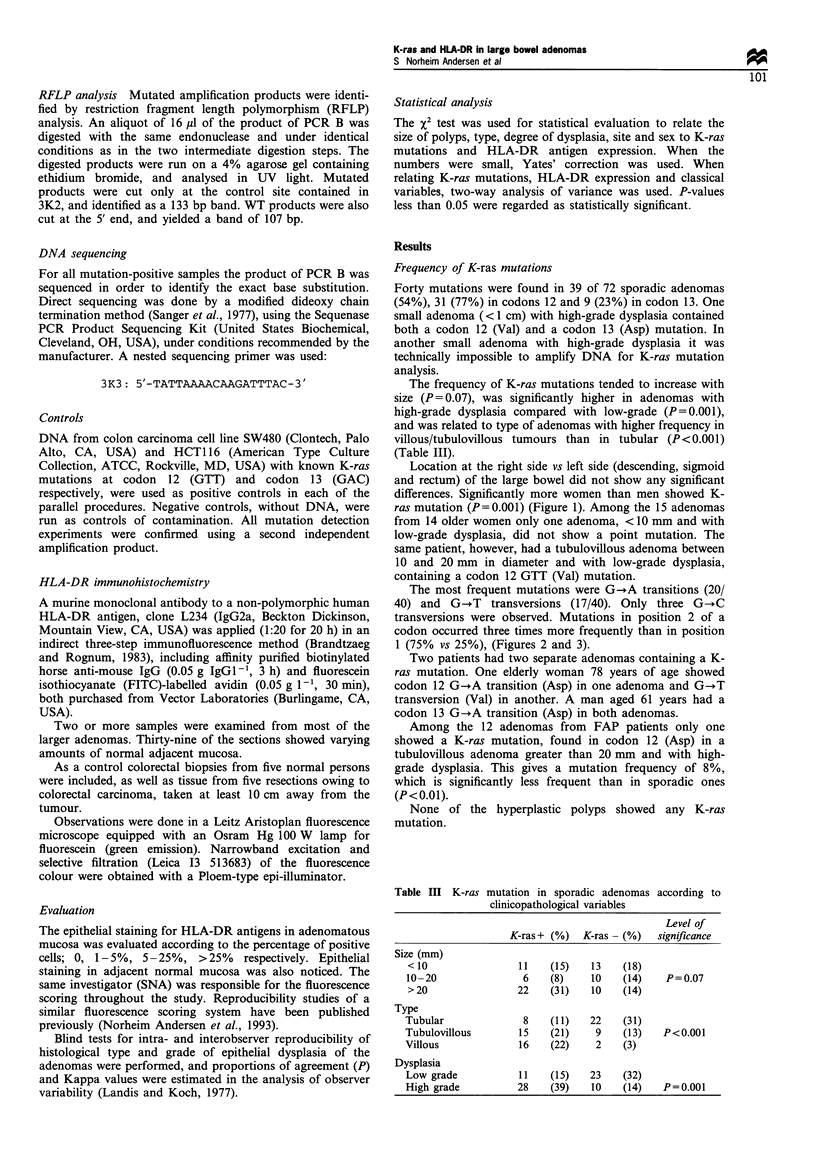
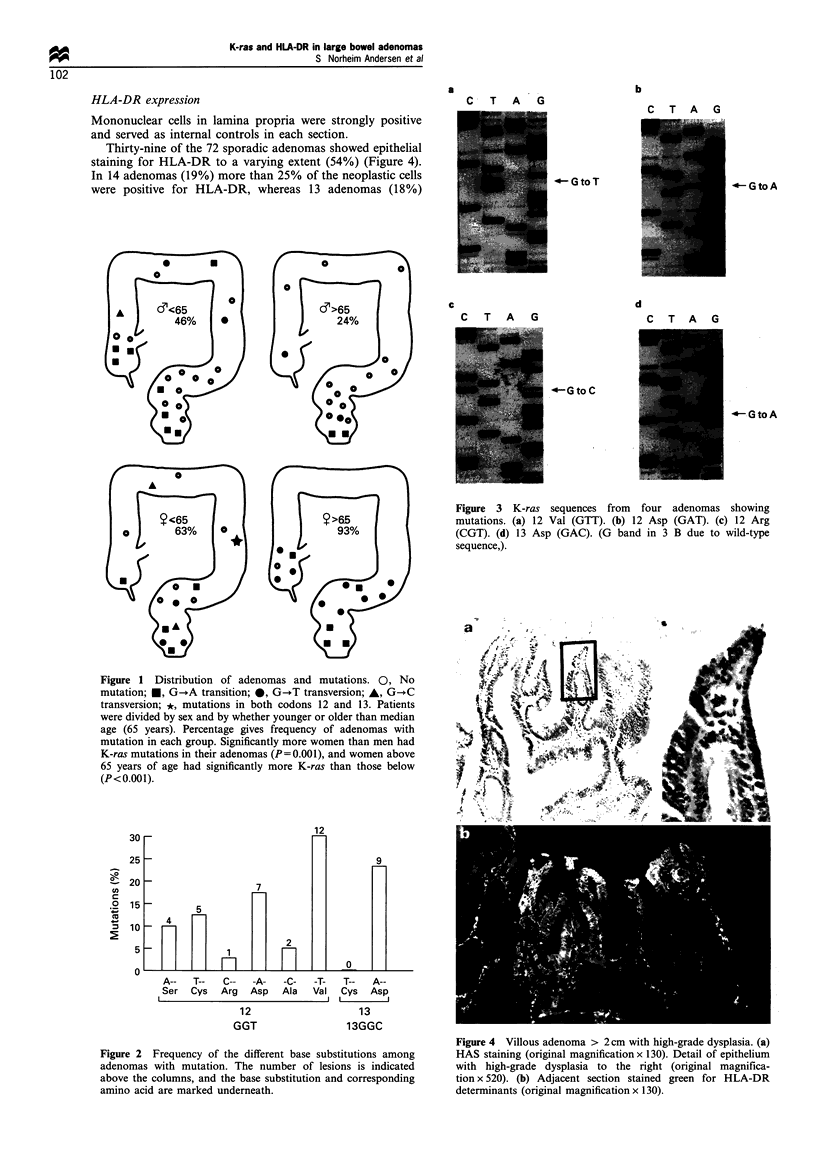
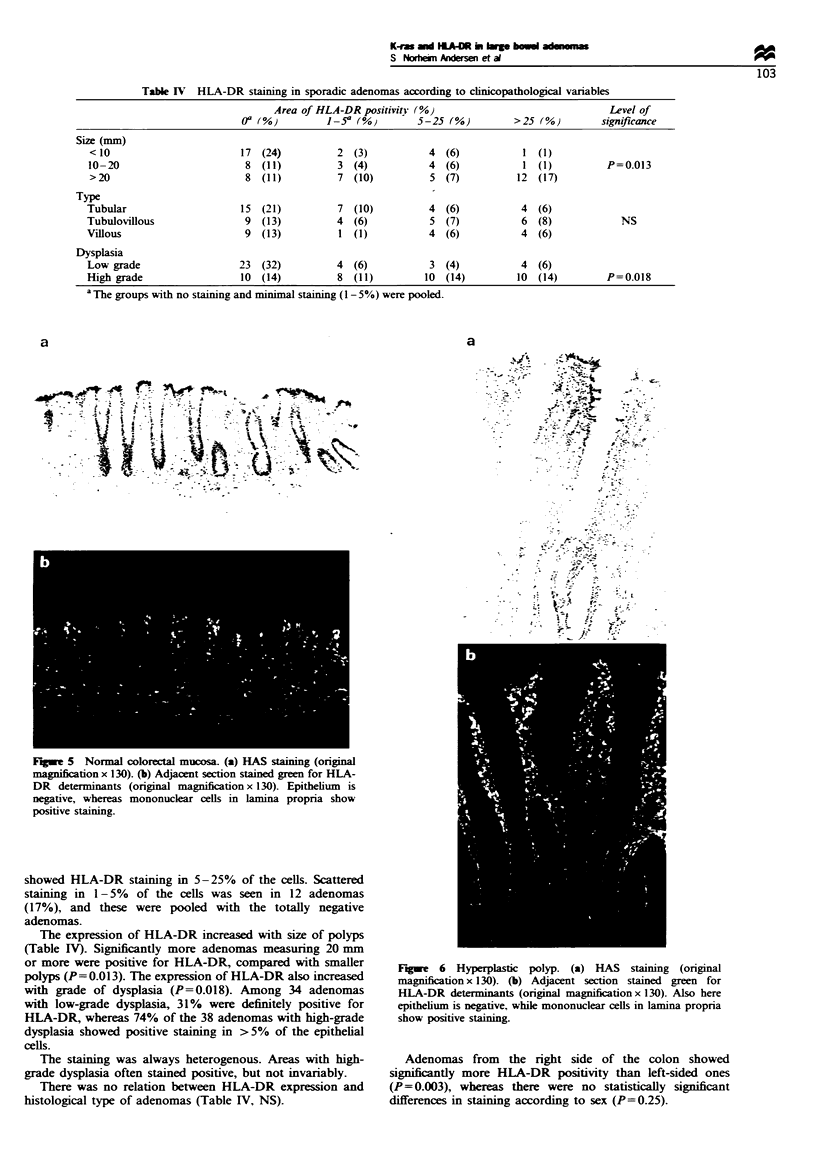
A total of 72 sporadic colorectal adenomas in 56 patients were studied for the presence of point mutations in codons 12 and 13 of the K-ras gene and for HLA-DR antigen expression related to clinicopathological variables. Forty K-ras mutations in 39 adenomas were found (54%): 31 (77%) in codon 12 and nine (23%) in codon 13. There was a strong relationship between the incidence of K-ras mutations and adenoma type, degree of dysplasia and sex. The highest frequency of K-ras mutations was seen in large adenomas of the villous type with high-grade dysplasia. Fourteen out of 15 adenomas obtained from 14 women above 65 years of age carried mutations. HLA-DR positivity was found in 38% of the adenomas, large tumours and those with high-grade dysplasia having the strongest staining. Coexpression of K-ras mutations and HLA-DR was found significantly more frequently in large and highly dysplastic adenomas, although two-way analysis of variance showing size and grade of dysplasia to be the most important variable. None of the adenomas with low-grade dysplasia showed both K-ras mutation and HLA-DR positivity (P = 0.004). K-ras mutation is recognised as an early event in colorectal carcinogenesis. The mutation might give rise to peptides that may be presented on the tumour cell surface by class II molecules, and thereby induce immune responses against neoplastic cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen S. N., Rognum T. O., Lund E., Meling G. I., Hauge S. Strong HLA-DR expression in large bowel carcinomas is associated with good prognosis. Br J Cancer. 1993 Jul;68(1):80–85. doi: 10.1038/bjc.1993.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando M., Maruyama M., Oto M., Takemura K., Endo M., Yuasa Y. Higher frequency of point mutations in the c-K-ras 2 gene in human colorectal adenomas with severe atypia than in carcinomas. Jpn J Cancer Res. 1991 Mar;82(3):245–249. doi: 10.1111/j.1349-7006.1991.tb01836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando M., Takemura K., Maruyama M., Endo M., Iwama T., Yuasa Y. Mutations in c-K-ras 2 gene codon 12 during colorectal tumorigenesis in familial adenomatous polyposis. Gastroenterology. 1992 Dec;103(6):1725–1731. doi: 10.1016/0016-5085(92)91427-6. [DOI] [PubMed] [Google Scholar]
- Bedossa P., Poynard T., Bacci J., Naveau S., Lemaigre G., Chaput J. C., Martin E. Expression of histocompatibility antigens and characterization of the lymphocyte infiltrate in hyperplastic polyps of the large bowel. Hum Pathol. 1990 Mar;21(3):319–324. doi: 10.1016/0046-8177(90)90233-u. [DOI] [PubMed] [Google Scholar]
- Bodmer W., Bishop T., Karran P. Genetic steps in colorectal cancer. Nat Genet. 1994 Mar;6(3):217–219. doi: 10.1038/ng0394-217. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components. Immunohistochemistry with a cold ethanol-fixation technique. Immunology. 1974 Jun;26(6):1101–1114. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Rognum T. O. Evaluation of tissue preparation methods and paired immunofluorescence staining for immunocytochemistry of lymphomas. Histochem J. 1983 Jul;15(7):655–689. doi: 10.1007/BF01002987. [DOI] [PubMed] [Google Scholar]
- Breivik J., Meling G. I., Spurkland A., Rognum T. O., Gaudernack G. K-ras mutation in colorectal cancer: relations to patient age, sex and tumour location. Br J Cancer. 1994 Feb;69(2):367–371. doi: 10.1038/bjc.1994.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmer G. C., Loeb L. A. Mutations in the KRAS2 oncogene during progressive stages of human colon carcinoma. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2403–2407. doi: 10.1073/pnas.86.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of MHC Class II antigens in normal human organs. Transplantation. 1984 Sep;38(3):293–298. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Ting A., Fabre J. W. Anomolous expression of HLA-DR antigens on human colorectal cancer cells. J Immunol. 1982 Aug;129(2):447–449. [PubMed] [Google Scholar]
- Degener T., Momburg F., Möller P. Differential expression of HLA-DR, HLA-DP, HLA-DQ and associated invariant chain (Ii) in normal colorectal mucosa, adenoma and carcinoma. Virchows Arch A Pathol Anat Histopathol. 1988;412(4):315–322. doi: 10.1007/BF00750257. [DOI] [PubMed] [Google Scholar]
- Farr C. J., Marshall C. J., Easty D. J., Wright N. A., Powell S. C., Paraskeva C. A study of ras gene mutations in colonic adenomas from familial polyposis coli patients. Oncogene. 1988 Dec;3(6):673–678. [PubMed] [Google Scholar]
- Forrester K., Almoguera C., Han K., Grizzle W. E., Perucho M. Detection of high incidence of K-ras oncogenes during human colon tumorigenesis. 1987 May 28-Jun 3Nature. 327(6120):298–303. doi: 10.1038/327298a0. [DOI] [PubMed] [Google Scholar]
- Fossum B., Gedde-Dahl T., 3rd, Breivik J., Eriksen J. A., Spurkland A., Thorsby E., Gaudernack G. p21-ras-peptide-specific T-cell responses in a patient with colorectal cancer. CD4+ and CD8+ T cells recognize a peptide corresponding to a common mutation (13Gly-->Asp). Int J Cancer. 1994 Jan 2;56(1):40–45. doi: 10.1002/ijc.2910560108. [DOI] [PubMed] [Google Scholar]
- Gedde-Dahl T., 3rd, Eriksen J. A., Thorsby E., Gaudernack G. T-cell responses against products of oncogenes: generation and characterization of human T-cell clones specific for p21 ras-derived synthetic peptides. Hum Immunol. 1992 Apr;33(4):266–274. doi: 10.1016/0198-8859(92)90334-j. [DOI] [PubMed] [Google Scholar]
- Gedde-Dahl T., 3rd, Fossum B., Eriksen J. A., Thorsby E., Gaudernack G. T cell clones specific for p21 ras-derived peptides: characterization of their fine specificity and HLA restriction. Eur J Immunol. 1993 Mar;23(3):754–760. doi: 10.1002/eji.1830230328. [DOI] [PubMed] [Google Scholar]
- Gedde-Dahl T., 3rd, Nilsen E., Thorsby E., Gaudernack G. Growth inhibition of a colonic adenocarcinoma cell line (HT29) by T cells specific for mutant p21 ras. Cancer Immunol Immunother. 1994 Feb;38(2):127–134. [PubMed] [Google Scholar]
- Gedde-Dahl T., 3rd, Spurkland A., Eriksen J. A., Thorsby E., Gaudernack G. Memory T cells of a patient with follicular thyroid carcinoma recognize peptides derived from mutated p21 ras (Gln-->Leu61). Int Immunol. 1992 Nov;4(11):1331–1337. doi: 10.1093/intimm/4.11.1331. [DOI] [PubMed] [Google Scholar]
- Ghosh A. K., Moore M., Street A. J., Howat J. M., Schofield P. F. Expression of HLA-D sub-region products on human colorectal carcinoma. Int J Cancer. 1986 Oct 15;38(4):459–464. doi: 10.1002/ijc.2910380402. [DOI] [PubMed] [Google Scholar]
- Gutierrez J., López-Nevot M. A., Cabrera T., Oliva R., Esquivias J., Ruiz-Cabello F., Garrido F. Class I and II HLA antigen distribution in normal mucosa, adenoma and colon carcinoma: relation with malignancy and invasiveness. Exp Clin Immunogenet. 1987;4(3):144–152. [PubMed] [Google Scholar]
- Gutierrez J., Ruiz-Cabello F., López Nevot M. A., Cabrera T., Esquivias J., Garrido F. Class II HLA antigen expression in familial polyposis coli is related to the degree of dysplasia. Immunobiology. 1990 Feb;180(2-3):138–148. doi: 10.1016/S0171-2985(11)80324-4. [DOI] [PubMed] [Google Scholar]
- Horie Y., Chiba M., Iizuka M., Igarashi K., Masamune O. HLA antigens on colorectal adenoma and cancer cells. Tohoku J Exp Med. 1990 Apr;160(4):311–322. doi: 10.1620/tjem.160.311. [DOI] [PubMed] [Google Scholar]
- Jen J., Powell S. M., Papadopoulos N., Smith K. J., Hamilton S. R., Vogelstein B., Kinzler K. W. Molecular determinants of dysplasia in colorectal lesions. Cancer Res. 1994 Nov 1;54(21):5523–5526. [PubMed] [Google Scholar]
- Jung S., Schluesener H. J. Human T lymphocytes recognize a peptide of single point-mutated, oncogenic ras proteins. J Exp Med. 1991 Jan 1;173(1):273–276. doi: 10.1084/jem.173.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S. M., Jiang W., Culbertson T. A., Weinstein I. B., Williams G. M., Tomita N., Ronai Z. Rapid and sensitive nonradioactive detection of mutant K-ras genes via 'enriched' PCR amplification. Oncogene. 1991 Jun;6(6):1079–1083. [PubMed] [Google Scholar]
- Kim H., Jen J., Vogelstein B., Hamilton S. R. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994 Jul;145(1):148–156. [PMC free article] [PubMed] [Google Scholar]
- Landis J. R., Koch G. G. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- Mayer L., Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987 Nov 1;166(5):1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall C. J., Ngoi S. S., Goldman I. S., Godwin T., Felix J., DeCosse J. J., Rigas B. Reduced expression of HLA class I and II antigens in colon cancer. Cancer Res. 1990 Dec 15;50(24):8023–8027. [PubMed] [Google Scholar]
- McLellan E. A., Owen R. A., Stepniewska K. A., Sheffield J. P., Lemoine N. R. High frequency of K-ras mutations in sporadic colorectal adenomas. Gut. 1993 Mar;34(3):392–396. doi: 10.1136/gut.34.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki M., Seki M., Okamoto M., Yamanaka A., Maeda Y., Tanaka K., Kikuchi R., Iwama T., Ikeuchi T., Tonomura A. Genetic changes and histopathological types in colorectal tumors from patients with familial adenomatous polyposis. Cancer Res. 1990 Nov 15;50(22):7166–7173. [PubMed] [Google Scholar]
- Momburg F., Degener T., Bacchus E., Moldenhauer G., Hämmerling G. J., Möller P. Loss of HLA-A,B,C and de novo expression of HLA-D in colorectal cancer. Int J Cancer. 1986 Feb 15;37(2):179–184. doi: 10.1002/ijc.2910370203. [DOI] [PubMed] [Google Scholar]
- Muto T., Bussey H. J., Morson B. C. The evolution of cancer of the colon and rectum. Cancer. 1975 Dec;36(6):2251–2270. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- Möller P., Koretz K., Schlag P., Momburg F. Frequency of abnormal expression of HLA-A,B,C and HLA-DR molecules, invariant chain, and LFA-3 (CD58) in colorectal carcinoma and its impact on tumor recurrence. Int J Cancer Suppl. 1991;6:155–162. doi: 10.1002/ijc.2910470727. [DOI] [PubMed] [Google Scholar]
- Norazmi M., Hohmann A. W., Skinner J. M., Bradley J. Expression of MHC class I and class II antigens in colonic carcinomas. Pathology. 1989 Oct;21(4):248–253. doi: 10.3109/00313028909061068. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Pretlow T. P., Brasitus T. A., Fulton N. C., Cheyer C., Kaplan E. L. K-ras mutations in putative preneoplastic lesions in human colon. J Natl Cancer Inst. 1993 Dec 15;85(24):2004–2007. doi: 10.1093/jnci/85.24.2004. [DOI] [PubMed] [Google Scholar]
- Rognum T. O., Brandtzaeg P., Elgjo K., Fausa O. Heterogeneous epithelial expression of class II (HLA-DR) determinants and secretory component related to dysplasia in ulcerative colitis. Br J Cancer. 1987 Oct;56(4):419–424. doi: 10.1038/bjc.1987.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognum T. O., Brandtzaeg P., Thorud E. Is heterogeneous expression of HLA-dr antigens and CEA along with DNA-profile variations evidence of phenotypic instability and clonal proliferation in human large bowel carcinomas? Br J Cancer. 1983 Oct;48(4):543–551. doi: 10.1038/bjc.1983.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognum T. O., Fausa O., Brandtzaeg P. Immunohistochemical evaluation of carcinoembryonic antigen, secretory component, and epithelial IgA in tubular and villous large-bowel adenomas with different grades of dysplasia. Scand J Gastroenterol. 1982 Apr;17(3):341–348. doi: 10.3109/00365528209182065. [DOI] [PubMed] [Google Scholar]
- Ruiz-Cabello F., Nevot M. A., Garrido F. MHC class I and II gene expression on human tumors. Adv Exp Med Biol. 1988;233:119–128. doi: 10.1007/978-1-4899-5037-6_14. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott N., Bell S. M., Sagar P., Blair G. E., Dixon M. F., Quirke P. p53 expression and K-ras mutation in colorectal adenomas. Gut. 1993 May;34(5):621–624. doi: 10.1136/gut.34.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh K., Yanagisawa A., Hiratsuka H., Sugano H., Kato Y. Variation in K-ras codon 12 point mutation rate with histological atypia within individual colorectal tumors. Jpn J Cancer Res. 1993 Apr;84(4):388–393. doi: 10.1111/j.1349-7006.1993.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsioulias G., Godwin T. A., Goldstein M. F., McDougall C. J., Ngoi S. S., DeCosse J. J., Rigas B. Loss of colonic HLA antigens in familial adenomatous polyposis. Cancer Res. 1992 Jun 15;52(12):3449–3452. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Minamoto T., Ochiai A., Onda M., Esumi H. Frequent and characteristic K-ras activation and absence of p53 protein accumulation in aberrant crypt foci of the colon. Gastroenterology. 1995 Feb;108(2):434–440. doi: 10.1016/0016-5085(95)90071-3. [DOI] [PubMed] [Google Scholar]