Abstract
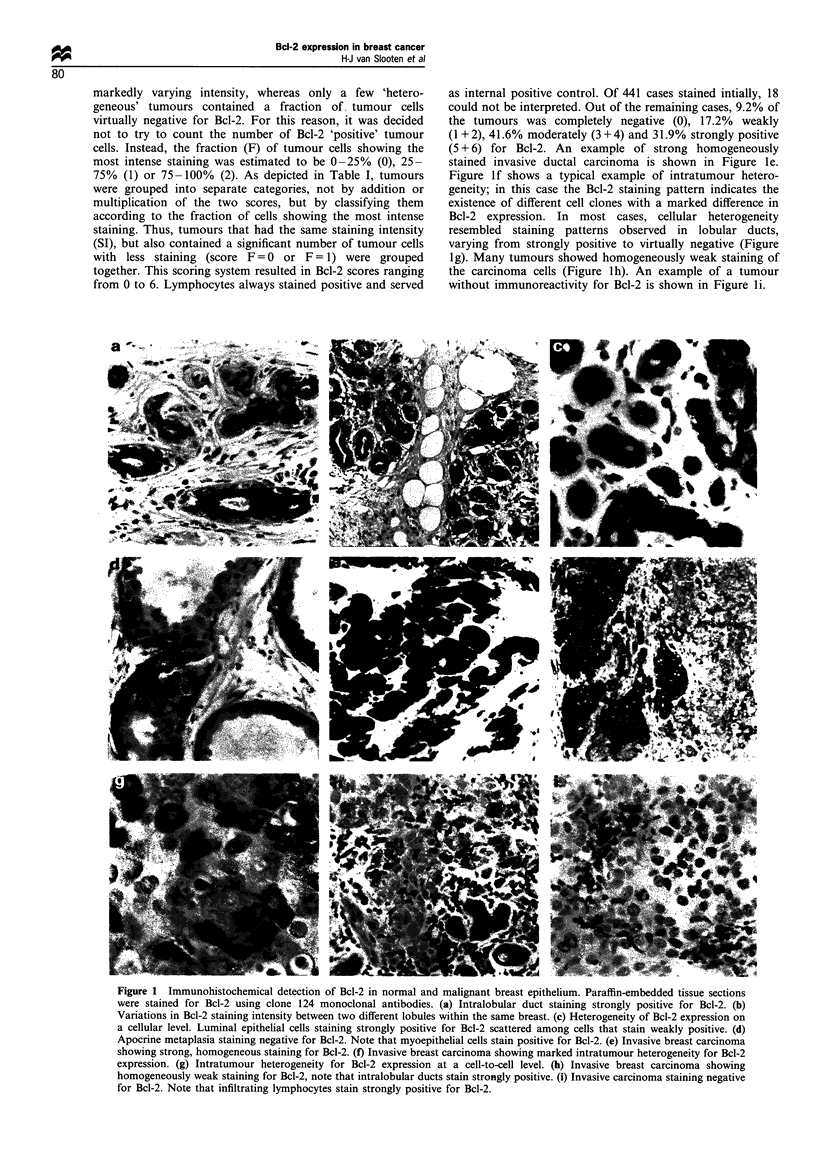
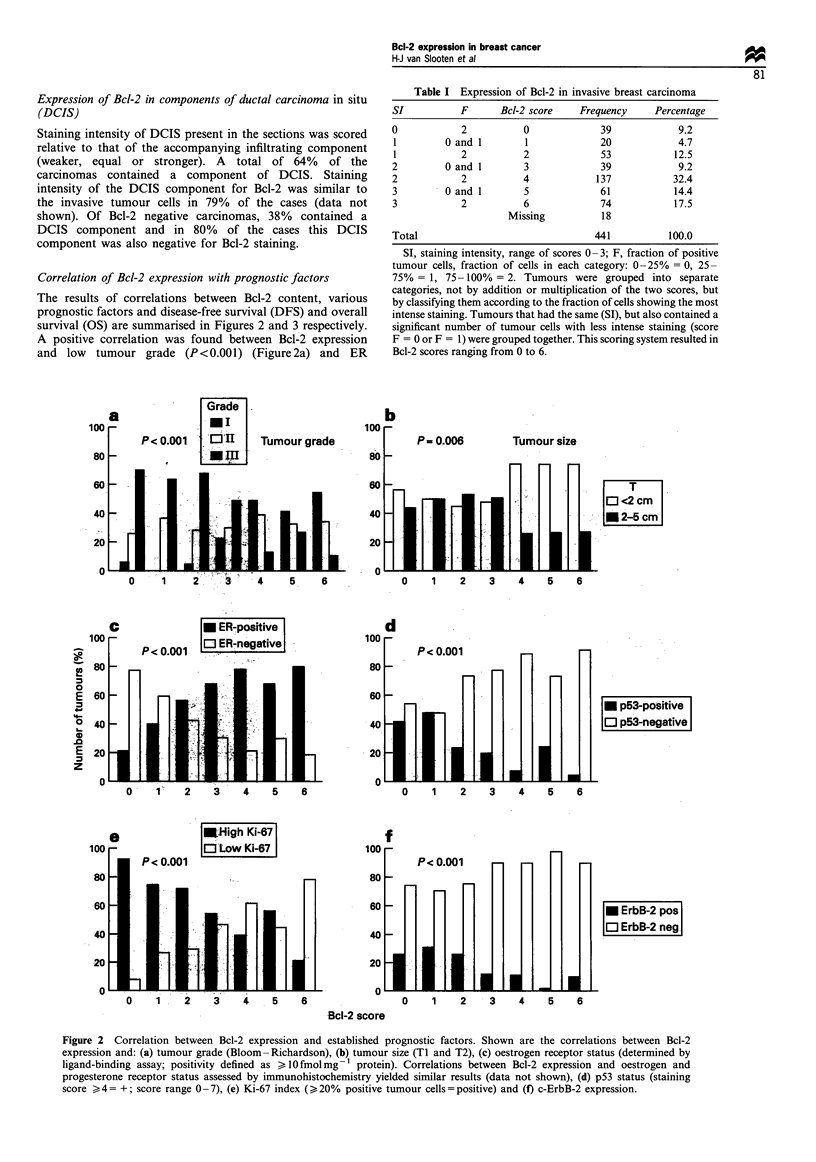
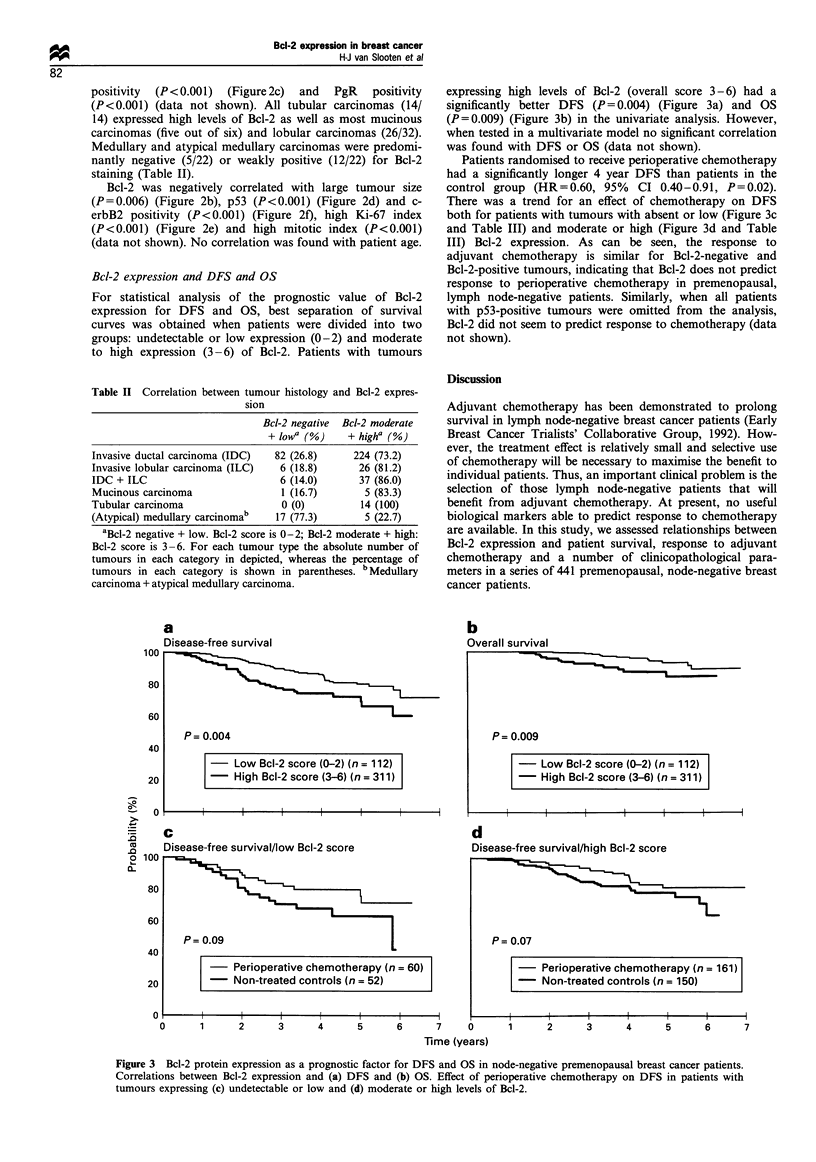
The aim of this study was to assess relationships between Bcl-2 expression, response to chemotherapy and a number of pathological and biological tumour parameters in premenopausal, lymph node-negative breast cancer patients. Expression of Bcl-2 was determined using immunohistochemistry on paraffin-embedded sections in a series of 441 premenopausal, lymph node-negative breast cancers of patients randomised to receive perioperative chemotherapy (5-fluorouracil, doxorubicin, cyclophosphamide) or no perioperative chemotherapy. Immunohistochemistry of Bcl-2 was evaluated by scoring both staining intensity (0-3) and number of positive cells (0-2). Using these scores tumours were grouped into categories 0-6. It was found that 9.2% of the tumours were completely negative (0), 17.2% weakly (1 + 2), 41.6% moderately (3 + 4) and 31.9% strongly positive (5 + 6) for Bcl-2. A positive correlation was found between high Bcl-2 expression and oestrogen (P < 0.001) and progesterone receptor positivity (P < 0.001) and low tumour grade (P < 0.001), whereas high Bcl-2 expression was negatively correlated with p53 (P < 0.001) and c-erb-B-2 positively (P < 0.001), high Ki-67 index (P < 0.001), mitotic index (P < 0.001) and large tumour size (P = 0.006). Patients with tumours expressing high levels of Bcl-2 (overall score 3-6) had a significantly better disease-free (P = 0.004) and overall (P = 0.009) survival. However, in a multivariate model this association no longer remained significant. There was a trend for an effect of adjuvant chemotherapy on disease-free survival both for patients with Bcl-2-positive (HR-0.61, 95% CI 0.35-1.06, P = 0.07) and negative (HR = 0.55, 95% CI 0.27-1.12, P = 0.09) breast tumours at a median follow-up of 49 months. The level of Bcl-2 expression does not seem to predict response to perioperative chemotherapy in premenopausal, lymph node-negative breast cancer patients. High levels of Bcl-2 are preferentially expressed in well-differentiated tumours and are associated with favourable prognosis. However, Bcl-2 expression is not an independent prognostic factor in this patient series.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhargava V., Kell D. L., van de Rijn M., Warnke R. A. Bcl-2 immunoreactivity in breast carcinoma correlates with hormone receptor positivity. Am J Pathol. 1994 Sep;145(3):535–540. [PMC free article] [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Campos L., Rouault J. P., Sabido O., Oriol P., Roubi N., Vasselon C., Archimbaud E., Magaud J. P., Guyotat D. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993 Jun 1;81(11):3091–3096. [PubMed] [Google Scholar]
- Castle V. P., Heidelberger K. P., Bromberg J., Ou X., Dole M., Nuñez G. Expression of the apoptosis-suppressing protein bcl-2, in neuroblastoma is associated with unfavorable histology and N-myc amplification. Am J Pathol. 1993 Dec;143(6):1543–1550. [PMC free article] [PubMed] [Google Scholar]
- Civitareale D., Lonigro R., Sinclair A. J., Di Lauro R. A thyroid-specific nuclear protein essential for tissue-specific expression of the thyroglobulin promoter. EMBO J. 1989 Sep;8(9):2537–2542. doi: 10.1002/j.1460-2075.1989.tb08391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clahsen P. C., van de Velde C. J., Julien J. P., Floiras J. L., Mignolet F. Y. Thromboembolic complications after perioperative chemotherapy in women with early breast cancer: a European Organization for Research and Treatment of Cancer Breast Cancer Cooperative Group study. J Clin Oncol. 1994 Jun;12(6):1266–1271. doi: 10.1200/JCO.1994.12.6.1266. [DOI] [PubMed] [Google Scholar]
- Debbas M., White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993 Apr;7(4):546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- Gasparini G., Barbareschi M., Doglioni C., Palma P. D., Mauri F. A., Boracchi P., Bevilacqua P., Caffo O., Morelli L., Verderio P. Expression of bcl-2 protein predicts efficacy of adjuvant treatments in operable node-positive breast cancer. Clin Cancer Res. 1995 Feb;1(2):189–198. [PubMed] [Google Scholar]
- Gee J. M., Robertson J. F., Ellis I. O., Willsher P., McClelland R. A., Hoyle H. B., Kyme S. R., Finlay P., Blamey R. W., Nicholson R. I. Immunocytochemical localization of BCL-2 protein in human breast cancers and its relationship to a series of prognostic markers and response to endocrine therapy. Int J Cancer. 1994 Dec 1;59(5):619–628. doi: 10.1002/ijc.2910590508. [DOI] [PubMed] [Google Scholar]
- Gompel A., Sabourin J. C., Martin A., Yaneva H., Audouin J., Decroix Y., Poitout P. Bcl-2 expression in normal endometrium during the menstrual cycle. Am J Pathol. 1994 Jun;144(6):1195–1202. [PMC free article] [PubMed] [Google Scholar]
- Haldar S., Negrini M., Monne M., Sabbioni S., Croce C. M. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994 Apr 15;54(8):2095–2097. [PubMed] [Google Scholar]
- Hellemans P., van Dam P. A., Weyler J., van Oosterom A. T., Buytaert P., Van Marck E. Prognostic value of bcl-2 expression in invasive breast cancer. Br J Cancer. 1995 Aug;72(2):354–360. doi: 10.1038/bjc.1995.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola J., Visakorpi T., Holli K., Kallioniemi O. P. Association of overexpression of tumor suppressor protein p53 with rapid cell proliferation and poor prognosis in node-negative breast cancer patients. J Natl Cancer Inst. 1992 Jul 15;84(14):1109–1114. doi: 10.1093/jnci/84.14.1109. [DOI] [PubMed] [Google Scholar]
- Joensuu H., Pylkkänen L., Toikkanen S. Bcl-2 protein expression and long-term survival in breast cancer. Am J Pathol. 1994 Nov;145(5):1191–1198. [PMC free article] [PubMed] [Google Scholar]
- Kiefer M. C., Brauer M. J., Powers V. C., Wu J. J., Umansky S. R., Tomei L. D., Barr P. J. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995 Apr 20;374(6524):736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- Leek R. D., Kaklamanis L., Pezzella F., Gatter K. C., Harris A. L. bcl-2 in normal human breast and carcinoma, association with oestrogen receptor-positive, epidermal growth factor receptor-negative tumours and in situ cancer. Br J Cancer. 1994 Jan;69(1):135–139. doi: 10.1038/bjc.1994.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. Y., Orlofsky A., Berger M. S., Prystowsky M. B. Characterization of A1, a novel hemopoietic-specific early-response gene with sequence similarity to bcl-2. J Immunol. 1993 Aug 15;151(4):1979–1988. [PubMed] [Google Scholar]
- Lowe S. W., Bodis S., McClatchey A., Remington L., Ruley H. E., Fisher D. E., Housman D. E., Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994 Nov 4;266(5186):807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- Merritt A. J., Potten C. S., Kemp C. J., Hickman J. A., Balmain A., Lane D. P., Hall P. A. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994 Feb 1;54(3):614–617. [PubMed] [Google Scholar]
- Miyashita T., Harigai M., Hanada M., Reed J. C. Identification of a p53-dependent negative response element in the bcl-2 gene. Cancer Res. 1994 Jun 15;54(12):3131–3135. [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Turley H., Kuzu I., Tungekar M. F., Dunnill M. S., Pierce C. B., Harris A., Gatter K. C., Mason D. Y. bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med. 1993 Sep 2;329(10):690–694. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]
- Pilotti S., Collini P., Rilke F., Cattoretti G., Del Bo R., Pierotti M. A. Bcl-2 protein expression in carcinomas originating from the follicular epithelium of the thyroid gland. J Pathol. 1994 Apr;172(4):337–342. doi: 10.1002/path.1711720408. [DOI] [PubMed] [Google Scholar]
- Reynolds J. E., Yang T., Qian L., Jenkinson J. D., Zhou P., Eastman A., Craig R. W. Mcl-1, a member of the Bcl-2 family, delays apoptosis induced by c-Myc overexpression in Chinese hamster ovary cells. Cancer Res. 1994 Dec 15;54(24):6348–6352. [PubMed] [Google Scholar]
- Selvakumaran M., Lin H. K., Miyashita T., Wang H. G., Krajewski S., Reed J. C., Hoffman B., Liebermann D. Immediate early up-regulation of bax expression by p53 but not TGF beta 1: a paradigm for distinct apoptotic pathways. Oncogene. 1994 Jun;9(6):1791–1798. [PubMed] [Google Scholar]
- Silvestrini R., Veneroni S., Daidone M. G., Benini E., Boracchi P., Mezzetti M., Di Fronzo G., Rilke F., Veronesi U. The Bcl-2 protein: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. J Natl Cancer Inst. 1994 Apr 6;86(7):499–504. doi: 10.1093/jnci/86.7.499. [DOI] [PubMed] [Google Scholar]
- Yang E., Zha J., Jockel J., Boise L. H., Thompson C. B., Korsmeyer S. J. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995 Jan 27;80(2):285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yin X. M., Oltvai Z. N., Korsmeyer S. J. Heterodimerization with Bax is required for Bcl-2 to repress cell death. Curr Top Microbiol Immunol. 1995;194:331–338. doi: 10.1007/978-3-642-79275-5_38. [DOI] [PubMed] [Google Scholar]
- Zhu Y. M., Bradbury D. A., Russell N. H. Wild-type p53 is required for apoptosis induced by growth factor deprivation in factor-dependent leukaemic cells. Br J Cancer. 1994 Mar;69(3):468–472. doi: 10.1038/bjc.1994.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong D., Prins F. A., Mason D. Y., Reed J. C., van Ommen G. B., Kluin P. M. Subcellular localization of the bcl-2 protein in malignant and normal lymphoid cells. Cancer Res. 1994 Jan 1;54(1):256–260. [PubMed] [Google Scholar]
- van de Vijver M. J., Peterse J. L., Mooi W. J., Wisman P., Lomans J., Dalesio O., Nusse R. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988 Nov 10;319(19):1239–1245. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]



