Abstract
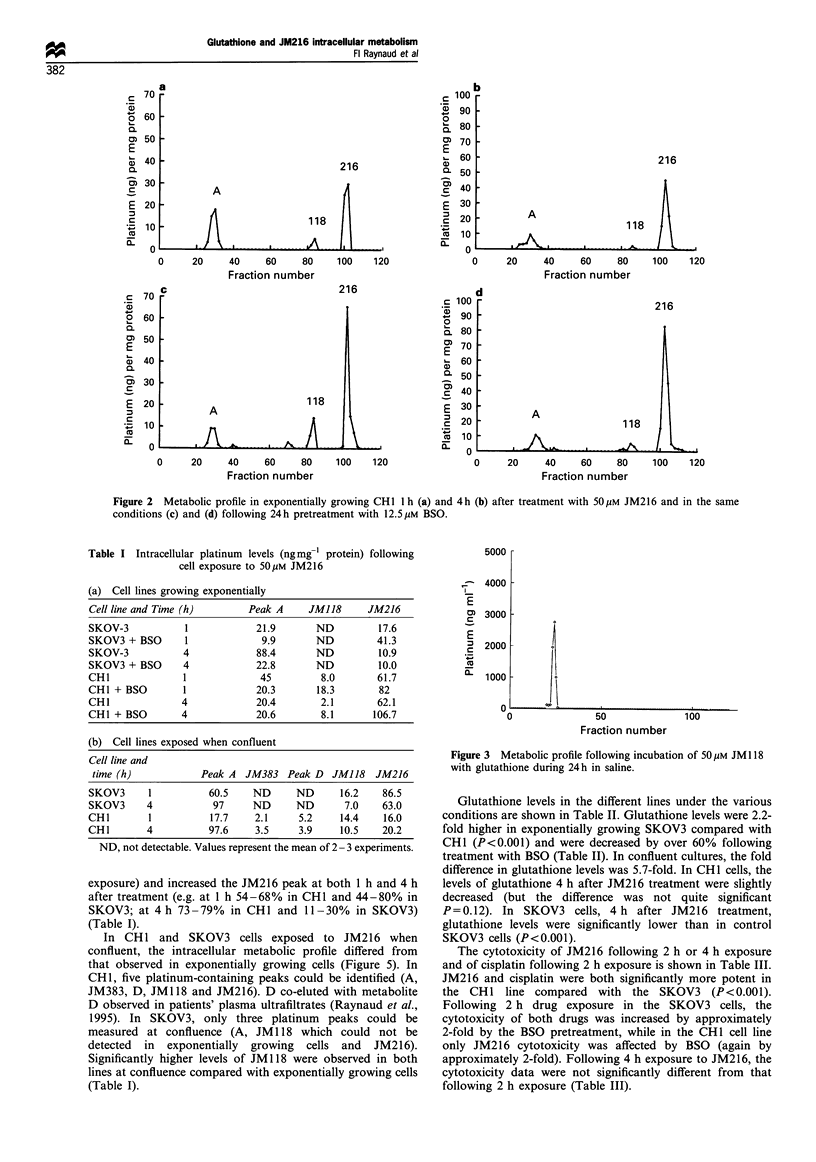
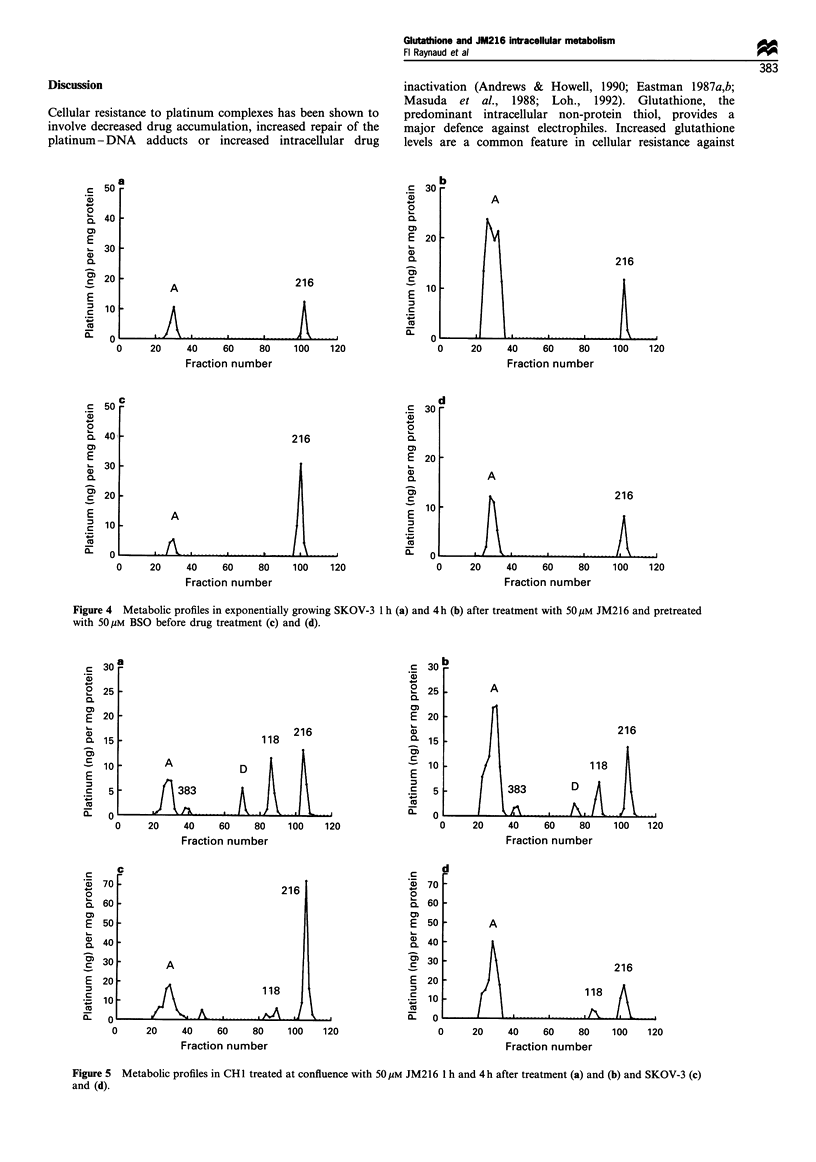
JM216 (bis-acetato ammine dichloro cyclohexylamine Pt IV) is an oral platinum complex presently undergoing phase II clinical trials. Previous studies have identified some of its biotransformation products in clinical materials. This study evaluated the nature of JM216 biotransformation products intracellularly in two different human ovarian carcinoma cell lines, one relatively sensitive to platinum agents (CH1: JM216 4 h IC50 of 5.8 microM) and the other relatively resistant (SKOV3: JM216 4 h IC50 of 60.7 microM). Metabolic profiles were also evaluated at different growth status and in cells pretreated with buthionine sulphoximine (BSO), an agent known to decrease intracellular glutathione levels. Results showed that JM216 enters the cells and that the nature and percentage of biotransformation products was dependent upon glutathione levels. Furthermore, results support the view that the previously reported peak A biotransformation product contains a glutathione adduct. In exponentially growing SKOV3 cells which contain higher glutathione levels than CH1, (82.5 vs 37.8 nmol mg-1 protein), peak A represented 89% of total platinum 4 h after JM216 exposure compared with only 24% in CH1. Moreover, 60-70% depletion of glutathione achieved by 24 h pretreatment of cells with BSO resulted in a significant decrease in peak A in both cell lines and increased the cytotoxicity of JM216 in both CH1 and SKOV3 by approximately 2-fold. Following a 4 h exposure of exponentially growing SKOV3 cells to JM216, only peak A (89%) and JM216 (11%) could be detected whereas in CH1 cells, peak A (24%), JM216 (73%) and JM118 [cis-ammine dichloro (cyclohexylamine) platinum II] (3%) were detected. However, in CH1 cells at confluence, where glutathione is lower (8 nmol mg-1 protein) four metabolites (plus JM216 itself) were detected following exposure to 50 microM JM216; peak A, JM118, JM383 (bis-acetato ammine (cyclohexylamine) dihydroxy platinum IV) and an unidentified metabolite (D), also observed in patient's plasma ultrafiltrate. In confluent SKOV3 cells exposed to 50 microM JM216, peak A, JM216 and JM118 were detected. A further unidentified metabolite observed in patients receiving JM216 (metabolite F) was not formed inside these tumour cells. Overall, these data suggest that glutathione conjugation represents a major deactivation pathway for JM216.
Full text
PDF
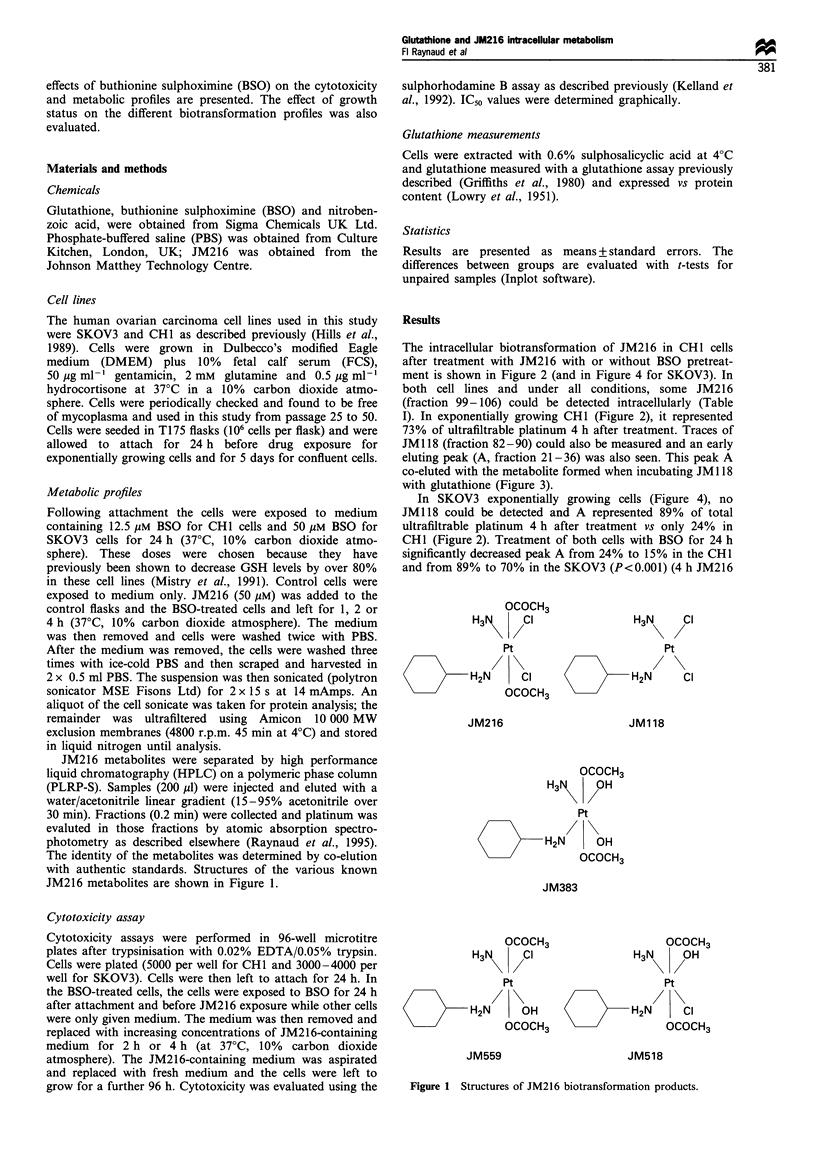



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. A., Howell S. B. Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells. 1990 Feb;2(2):35–43. [PubMed] [Google Scholar]
- Andrews P. A., Murphy M. P., Howell S. B. Differential potentiation of alkylating and platinating agent cytotoxicity in human ovarian carcinoma cells by glutathione depletion. Cancer Res. 1985 Dec;45(12 Pt 1):6250–6253. [PubMed] [Google Scholar]
- Andrews P. A., Schiefer M. A., Murphy M. P., Howell S. B. Enhanced potentiation of cisplatin cytotoxicity in human ovarian carcinoma cells by prolonged glutathione depletion. Chem Biol Interact. 1988;65(1):51–58. doi: 10.1016/0009-2797(88)90030-0. [DOI] [PubMed] [Google Scholar]
- Batist G., Behrens B. C., Makuch R., Hamilton T. C., Katki A. G., Louie K. G., Myers C. E., Ozols R. F. Serial determinations of glutathione levels and glutathione-related enzyme activities in human tumor cells in vitro. Biochem Pharmacol. 1986 Jul 1;35(13):2257–2259. doi: 10.1016/0006-2952(86)90601-5. [DOI] [PubMed] [Google Scholar]
- Eastman A. Glutathione-mediated activation of anticancer platinum(IV) complexes. Biochem Pharmacol. 1987 Dec 1;36(23):4177–4178. doi: 10.1016/0006-2952(87)90581-8. [DOI] [PubMed] [Google Scholar]
- Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- Gibbons G. R., Wyrick S., Chaney S. G. Rapid reduction of tetrachloro(D,L-trans)1,2-diaminocyclohexaneplatinum(IV) (tetraplatin) in RPMI 1640 tissue culture medium. Cancer Res. 1989 Mar 15;49(6):1402–1407. [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Hills C. A., Kelland L. R., Abel G., Siracky J., Wilson A. P., Harrap K. R. Biological properties of ten human ovarian carcinoma cell lines: calibration in vitro against four platinum complexes. Br J Cancer. 1989 Apr;59(4):527–534. doi: 10.1038/bjc.1989.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosking L. K., Whelan R. D., Shellard S. A., Bedford P., Hill B. T. An evaluation of the role of glutathione and its associated enzymes in the expression of differential sensitivities to antitumour agents shown by a range of human tumour cell lines. Biochem Pharmacol. 1990 Oct 15;40(8):1833–1842. doi: 10.1016/0006-2952(90)90364-q. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem. 1993 Sep 25;268(27):20116–20125. [PubMed] [Google Scholar]
- Ishikawa T., Wright C. D., Ishizuka H. GS-X pump is functionally overexpressed in cis-diamminedichloroplatinum (II)-resistant human leukemia HL-60 cells and down-regulated by cell differentiation. J Biol Chem. 1994 Nov 18;269(46):29085–29093. [PubMed] [Google Scholar]
- Kelland L. R., Abel G., McKeage M. J., Jones M., Goddard P. M., Valenti M., Murrer B. A., Harrap K. R. Preclinical antitumor evaluation of bis-acetato-ammine-dichloro-cyclohexylamine platinum(IV): an orally active platinum drug. Cancer Res. 1993 Jun 1;53(11):2581–2586. [PubMed] [Google Scholar]
- Kelland L. R., Murrer B. A., Abel G., Giandomenico C. M., Mistry P., Harrap K. R. Ammine/amine platinum(IV) dicarboxylates: a novel class of platinum complex exhibiting selective cytotoxicity to intrinsically cisplatin-resistant human ovarian carcinoma cell lines. Cancer Res. 1992 Feb 15;52(4):822–828. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis A. D., Hayes J. D., Wolf C. R. Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: intrinsic differences and cell cycle effects. Carcinogenesis. 1988 Jul;9(7):1283–1287. doi: 10.1093/carcin/9.7.1283. [DOI] [PubMed] [Google Scholar]
- Loh S. Y., Mistry P., Kelland L. R., Abel G., Harrap K. R. Reduced drug accumulation as a major mechanism of acquired resistance to cisplatin in a human ovarian carcinoma cell line: circumvention studies using novel platinum (II) and (IV) ammine/amine complexes. Br J Cancer. 1992 Dec;66(6):1109–1115. doi: 10.1038/bjc.1992.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Ozols R. F., Lai G. M., Fojo A., Rothenberg M., Hamilton T. C. Increased DNA repair as a mechanism of acquired resistance to cis-diamminedichloroplatinum (II) in human ovarian cancer cell lines. Cancer Res. 1988 Oct 15;48(20):5713–5716. [PubMed] [Google Scholar]
- McKeage M. J., Boxall F. E., Jones M., Harrap K. R. Lack of neurotoxicity of oral bisacetatoamminedichlorocyclohexylamine-platinum(IV) in comparison to cisplatin and tetraplatin in the rat. Cancer Res. 1994 Feb 1;54(3):629–631. [PubMed] [Google Scholar]
- McKeage M. J., Kelland L. R., Boxall F. E., Valenti M. R., Jones M., Goddard P. M., Gwynne J., Harrap K. R. Schedule dependency of orally administered bis-acetato-ammine-dichloro-cyclohexylamine-platinum(IV) (JM216) in vivo. Cancer Res. 1994 Aug 1;54(15):4118–4122. [PubMed] [Google Scholar]
- McKeage M. J., Mistry P., Ward J., Boxall F. E., Loh S., O'Neill C., Ellis P., Kelland L. R., Morgan S. E., Murrer B. A phase I and pharmacology study of an oral platinum complex, JM216: dose-dependent pharmacokinetics with single-dose administration. Cancer Chemother Pharmacol. 1995;36(6):451–458. doi: 10.1007/BF00685793. [DOI] [PubMed] [Google Scholar]
- McKeage M. J., Morgan S. E., Boxall F. E., Murrer B. A., Hard G. C., Harrap K. R. Lack of nephrotoxicity of oral ammine/amine platinum (IV) dicarboxylate complexes in rodents. Br J Cancer. 1993 May;67(5):996–1000. doi: 10.1038/bjc.1993.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer C., Mulder N. H., Timmer-Bosscha H., Sluiter W. J., Meersma G. J., de Vries E. G. Relationship of cellular glutathione to the cytotoxicity and resistance of seven platinum compounds. Cancer Res. 1992 Dec 15;52(24):6885–6889. [PubMed] [Google Scholar]
- Mellish K. J., Barnard C. F., Murrer B. A., Kelland L. R. DNA-binding properties of novel cis- and trans platinum-based anticancer agents in 2 human ovarian carcinoma cell lines. Int J Cancer. 1995 Sep 15;62(6):717–723. doi: 10.1002/ijc.2910620612. [DOI] [PubMed] [Google Scholar]
- Mellish K. J., Kelland L. R., Harrap K. R. In vitro platinum drug chemosensitivity of human cervical squamous cell carcinoma cell lines with intrinsic and acquired resistance to cisplatin. Br J Cancer. 1993 Aug;68(2):240–250. doi: 10.1038/bjc.1993.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellish K. J., Kelland L. R. Mechanisms of acquired resistance to the orally active platinum-based anticancer drug bis-acetato-ammine-dichloro-cyclohexylamine platinum (i.v.) (JM216) in two human ovarian carcinoma cell lines. Cancer Res. 1994 Dec 1;54(23):6194–6200. [PubMed] [Google Scholar]
- Mistry P., Kelland L. R., Abel G., Sidhar S., Harrap K. R. The relationships between glutathione, glutathione-S-transferase and cytotoxicity of platinum drugs and melphalan in eight human ovarian carcinoma cell lines. Br J Cancer. 1991 Aug;64(2):215–220. doi: 10.1038/bjc.1991.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry P., Loh S. Y., Kelland L. R., Harrap K. R. Effect of buthionine sulfoximine on PtII and PtIV drug accumulation and the formation of glutathione conjugates in human ovarian-carcinoma cell lines. Int J Cancer. 1993 Nov 11;55(5):848–856. doi: 10.1002/ijc.2910550526. [DOI] [PubMed] [Google Scholar]
- Müller M., Meijer C., Zaman G. J., Borst P., Scheper R. J., Mulder N. H., de Vries E. G., Jansen P. L. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):13033–13037. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nováková O., Vrána O., Kiseleva V. I., Brabec V. DNA interactions of antitumor platinum(IV) complexes. Eur J Biochem. 1995 Mar 15;228(3):616–624. doi: 10.1111/j.1432-1033.1995.tb20301.x. [DOI] [PubMed] [Google Scholar]
- Orr R. M., O'Neill C. F., Nicolson M. C., Barnard C. F., Murrer B. A., Giandomenico C. M., Vollano J. F., Harrap K. R. Evaluation of novel ammine/amine platinum (IV) dicarboxylates in L1210 murine leukaemia cells sensitive and resistant to cisplatin, tetraplatin or carboplatin. Br J Cancer. 1994 Sep;70(3):415–420. doi: 10.1038/bjc.1994.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala L., Creaven P. J., Perez R., Zdanowicz J. R., Raghavan D. Intracellular glutathione and cytotoxicity of platinum complexes. Cancer Chemother Pharmacol. 1995;36(4):271–278. doi: 10.1007/BF00689042. [DOI] [PubMed] [Google Scholar]
- Pendyala L., Walsh J. R., Huq M. M., Arakali A. V., Cowens J. W., Creaven P. J. Uptake and metabolism of iproplatin in murine L1210 cells. Cancer Chemother Pharmacol. 1989;25(1):15–18. doi: 10.1007/BF00694332. [DOI] [PubMed] [Google Scholar]
- Poon G. K., Raynaud F. I., Mistry P., Odell D. E., Kelland L. R., Harrap K. R., Barnard C. F., Murrer B. A. Metabolic studies of an orally active platinum anticancer drug by liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr A. 1995 Sep 29;712(1):61–66. doi: 10.1016/0021-9673(95)00288-x. [DOI] [PubMed] [Google Scholar]
- Post G. B., Keller D. A., Connor K. A., Menzel D. B. Effects of culture conditions on glutathione content in A549 cells. Biochem Biophys Res Commun. 1983 Jul 29;114(2):737–742. doi: 10.1016/0006-291x(83)90842-2. [DOI] [PubMed] [Google Scholar]
- Richon V. M., Schulte N., Eastman A. Multiple mechanisms of resistance to cis-diamminedichloroplatinum(II) in murine leukemia L1210 cells. Cancer Res. 1987 Apr 15;47(8):2056–2061. [PubMed] [Google Scholar]
- Sharp S. Y., Rogers P. M., Kelland L. R. Transport of cisplatin and bis-acetato-ammine-dichlorocyclohexylamine Platinum(IV) (JM216) in human ovarian carcinoma cell lines: identification of a plasma membrane protein associated with cisplatin resistance. Clin Cancer Res. 1995 Sep;1(9):981–989. [PubMed] [Google Scholar]
- Twentyman P. R., Wright K. A., Mistry P., Kelland L. R., Murrer B. A. Sensitivity to novel platinum compounds of panels of human lung cancer cell lines with acquired and inherent resistance to cisplatin. Cancer Res. 1992 Oct 15;52(20):5674–5680. [PubMed] [Google Scholar]
- Versantvoort C. H., Broxterman H. J., Bagrij T., Scheper R. J., Twentyman P. R. Regulation by glutathione of drug transport in multidrug-resistant human lung tumour cell lines overexpressing multidrug resistance-associated protein. Br J Cancer. 1995 Jul;72(1):82–89. doi: 10.1038/bjc.1995.281. [DOI] [PMC free article] [PubMed] [Google Scholar]


