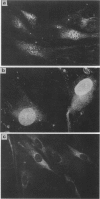
Abstract
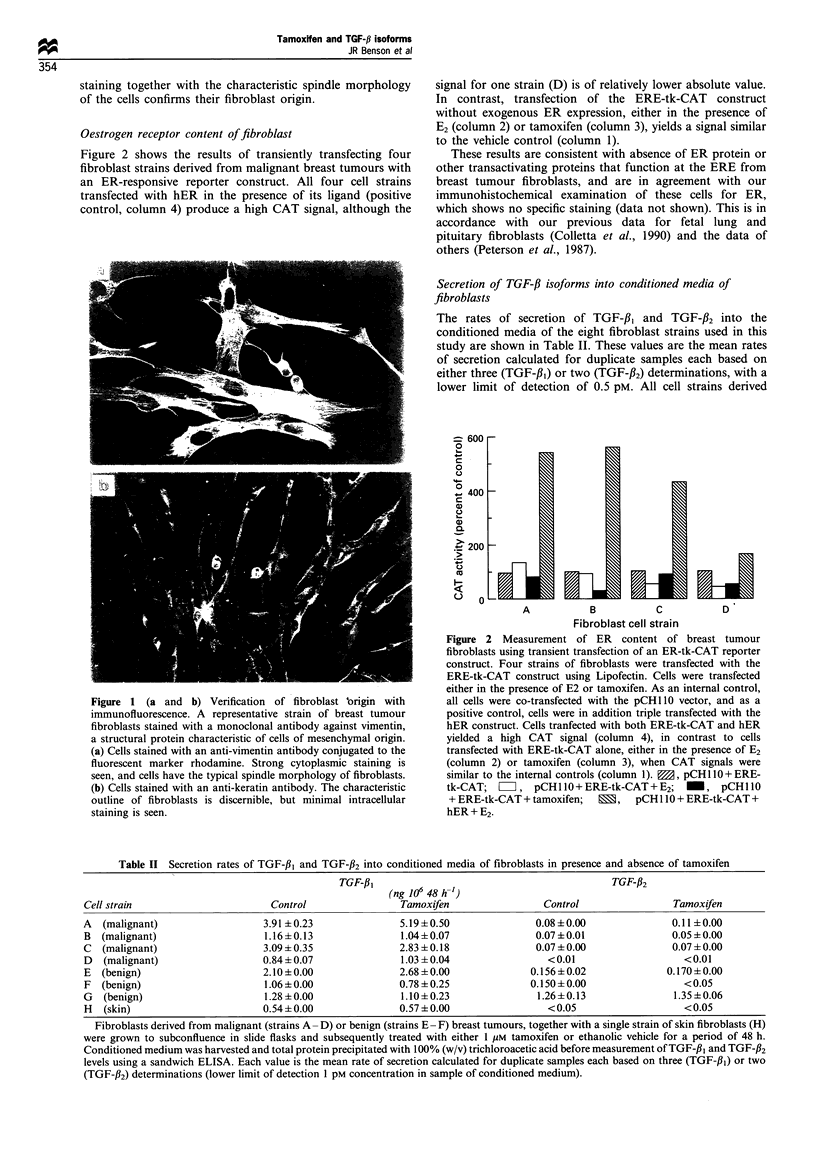
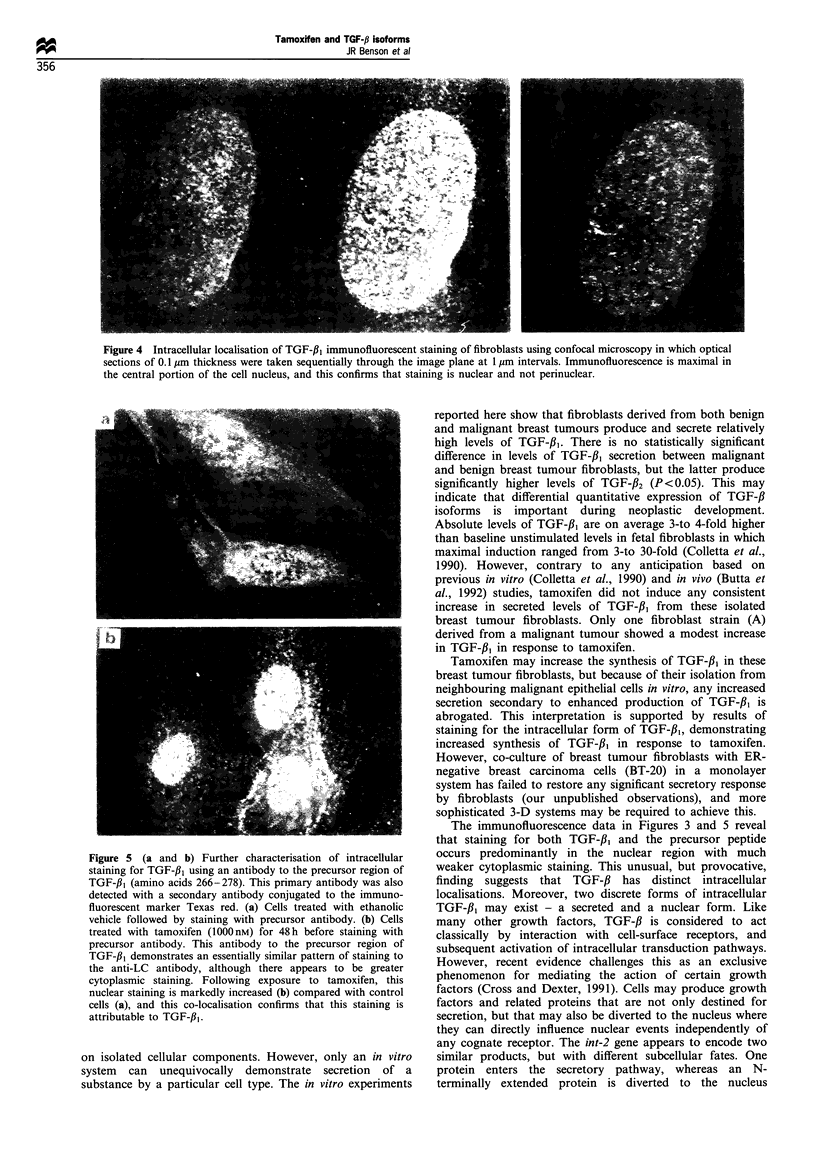
Tamoxifen may mediate its effect in early breast cancer in part via an oestrogen receptor (ER)-independent pathway by directly stimulating fibroblasts to produce the negative paracrine growth factor transforming growth factor (TGF)-beta. We have previously shown that secretion of this factor is induced 3-to 30-fold in human fetal fibroblasts in vitro, and by stromal fibroblasts in vivo following tamoxifen treatment of ER-positive and ER-negative breast cancer patients. Primary cultures of breast tumour fibroblasts have been exposed to tamoxifen for 48 h, and rates of secretion of TGF-beta 1 and TGF-beta 2 measured using a quantitative immunoassay. Fibroblast strains derived from malignant and benign tumours produced and secreted similar amounts of TGF-beta 1, but benign breast tumour fibroblasts secreted significantly higher levels of TGF-beta 2 compared with fibroblasts of malignant origin. Tamoxifen did not induce any consistent increase in TGF-beta secretion into the conditioned medium, but immunofluorescence analysis for the intracellular form of TGF-beta 1 revealed evidence of increased immunoreactive protein in tamoxifen-treated fibroblasts, which is localised to the nucleus. Therefore synthesis of TGF-beta 1 appears to be stimulated by tamoxifen, but increased secretion may be abrogated in vitro. Furthermore, using immunocytochemistry and transient transfection with an ER-responsive reporter construct, no ER was demonstrable in these fibroblasts supporting the proposed ER-independent paracrine pathway.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Adams E. F., Newton C. J., Braunsberg H., Shaikh N., Ghilchik M., James V. H. Effects of human breast fibroblasts on growth and 17 beta-estradiol dehydrogenase activity of MCF-7 cells in culture. Breast Cancer Res Treat. 1988 May;11(2):165–172. doi: 10.1007/BF01805840. [DOI] [PubMed] [Google Scholar]
- Arrick B. A., Grendell R. L., Griffin L. A. Enhanced translational efficiency of a novel transforming growth factor beta 3 mRNA in human breast cancer cells. Mol Cell Biol. 1994 Jan;14(1):619–628. doi: 10.1128/mcb.14.1.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. R., Baum M. Breast cancer, desmoid tumours, and familial adenomatous polyposis--a unifying hypothesis. Lancet. 1993 Oct 2;342(8875):848–850. doi: 10.1016/0140-6736(93)92700-4. [DOI] [PubMed] [Google Scholar]
- Brooks M. D., Ebbs S. R., Colletta A. A., Baum M. Desmoid tumours treated with triphenylethylenes. Eur J Cancer. 1992;28A(6-7):1014–1018. doi: 10.1016/0959-8049(92)90445-8. [DOI] [PubMed] [Google Scholar]
- Butta A., MacLennan K., Flanders K. C., Sacks N. P., Smith I., McKinna A., Dowsett M., Wakefield L. M., Sporn M. B., Baum M. Induction of transforming growth factor beta 1 in human breast cancer in vivo following tamoxifen treatment. Cancer Res. 1992 Aug 1;52(15):4261–4264. [PubMed] [Google Scholar]
- Colletta A. A., Wakefield L. M., Howell F. V., van Roozendaal K. E., Danielpour D., Ebbs S. R., Sporn M. B., Baum M. Anti-oestrogens induce the secretion of active transforming growth factor beta from human fetal fibroblasts. Br J Cancer. 1990 Sep;62(3):405–409. doi: 10.1038/bjc.1990.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M., Dexter T. M. Growth factors in development, transformation, and tumorigenesis. Cell. 1991 Jan 25;64(2):271–280. doi: 10.1016/0092-8674(91)90638-f. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Donjacour A. Stromal-epithelial interactions in normal and abnormal prostatic development. Prog Clin Biol Res. 1987;239:251–272. [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Flanders K. C., Thompson N. L., Cissel D. S., Van Obberghen-Schilling E., Baker C. C., Kass M. E., Ellingsworth L. R., Roberts A. B., Sporn M. B. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989 Feb;108(2):653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Haggie J. A., Sellwood R. A., Howell A., Birch J. M., Schor S. L. Fibroblasts from relatives of patients with hereditary breast cancer show fetal-like behaviour in vitro. Lancet. 1987 Jun 27;1(8548):1455–1457. doi: 10.1016/s0140-6736(87)92206-9. [DOI] [PubMed] [Google Scholar]
- Johnson L. K., Vlodavsky I., Baxter J. D., Gospodarowicz D. Nuclear accumulation of epidermal growth factor in cultured rat pituitary cells. Nature. 1980 Sep 25;287(5780):340–343. doi: 10.1038/287340a0. [DOI] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- MacCallum J., Bartlett J. M., Thompson A. M., Keen J. C., Dixon J. M., Miller W. R. Expression of transforming growth factor beta mRNA isoforms in human breast cancer. Br J Cancer. 1994 Jun;69(6):1006–1009. doi: 10.1038/bjc.1994.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir G. H., Butta A., Shearer R. J., Fisher C., Dearnaley D. P., Flanders K. C., Sporn M. B., Colletta A. A. Induction of transforming growth factor beta in hormonally treated human prostate cancer. Br J Cancer. 1994 Jan;69(1):130–134. doi: 10.1038/bjc.1994.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. R., Kang Y., Greaves B. R. Relationship between tamoxifen-induced transforming growth factor beta 1 expression, cytostasis and apoptosis in human breast cancer cells. Br J Cancer. 1995 Dec;72(6):1441–1446. doi: 10.1038/bjc.1995.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. W., Høyer P. E., van Deurs B. Frequency and distribution of estrogen receptor-positive cells in normal, nonlactating human breast tissue. Cancer Res. 1987 Nov 1;47(21):5748–5751. [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Flanders K. C., Kondaiah P., Thompson N. L., Van Obberghen-Schilling E., Wakefield L., Rossi P., de Crombrugghe B., Heine U., Sporn M. B. Transforming growth factor beta: biochemistry and roles in embryogenesis, tissue repair and remodeling, and carcinogenesis. Recent Prog Horm Res. 1988;44:157–197. doi: 10.1016/b978-0-12-571144-9.50010-7. [DOI] [PubMed] [Google Scholar]
- Spence A. M., Sheppard P. C., Davie J. R., Matuo Y., Nishi N., McKeehan W. L., Dodd J. G., Matusik R. J. Regulation of a bifunctional mRNA results in synthesis of secreted and nuclear probasin. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7843–7847. doi: 10.1073/pnas.86.20.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenius L. Two modes of interaction between oestrogen and anti-oestrogen. Acta Endocrinol (Copenh) 1970 May;64(1):47–58. doi: 10.1530/acta.0.0640047. [DOI] [PubMed] [Google Scholar]
- Tucker R. F., Branum E. L., Shipley G. D., Ryan R. J., Moses H. L. Specific binding to cultured cells of 125I-labeled type beta transforming growth factor from human platelets. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6757–6761. doi: 10.1073/pnas.81.21.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H. J., Pierce G. F., Deuel T. F. Ultrastructural localization of a platelet-derived growth factor/v-sis-related protein(s) in cytoplasm and nucleus of simian sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2317–2321. doi: 10.1073/pnas.84.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]