Abstract
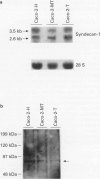
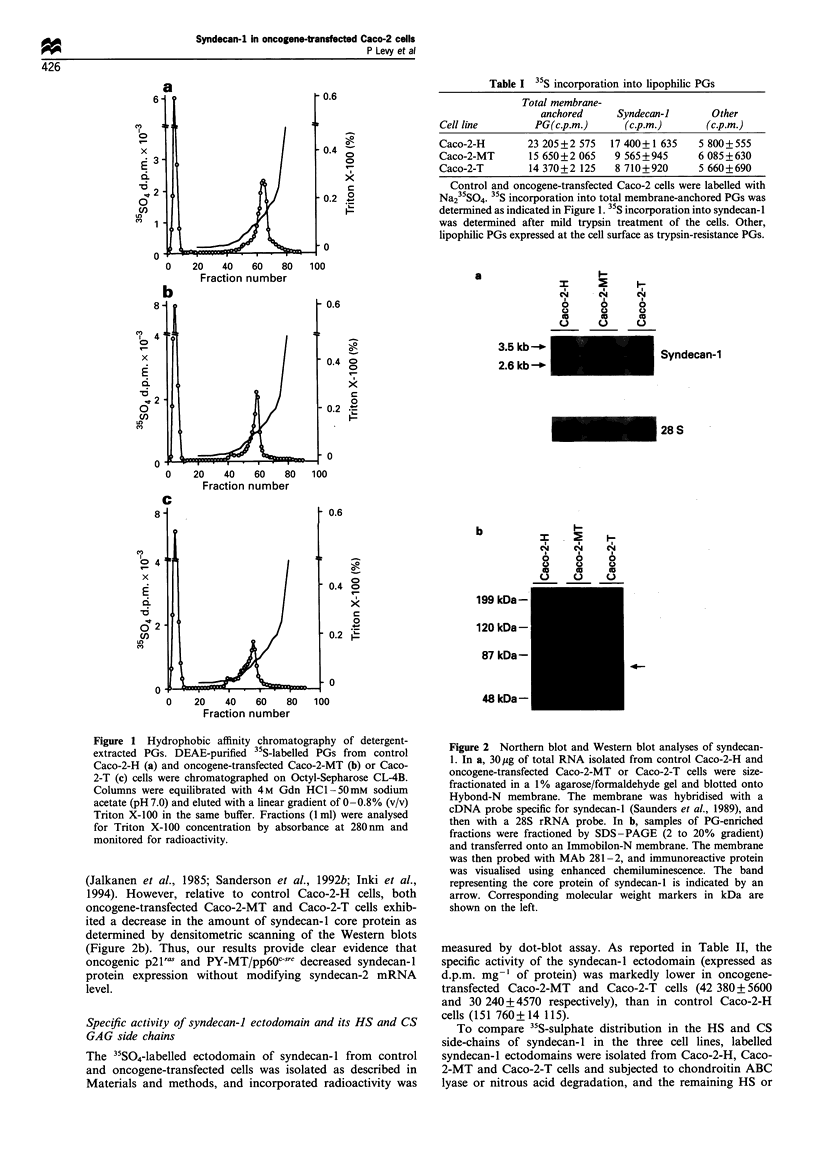
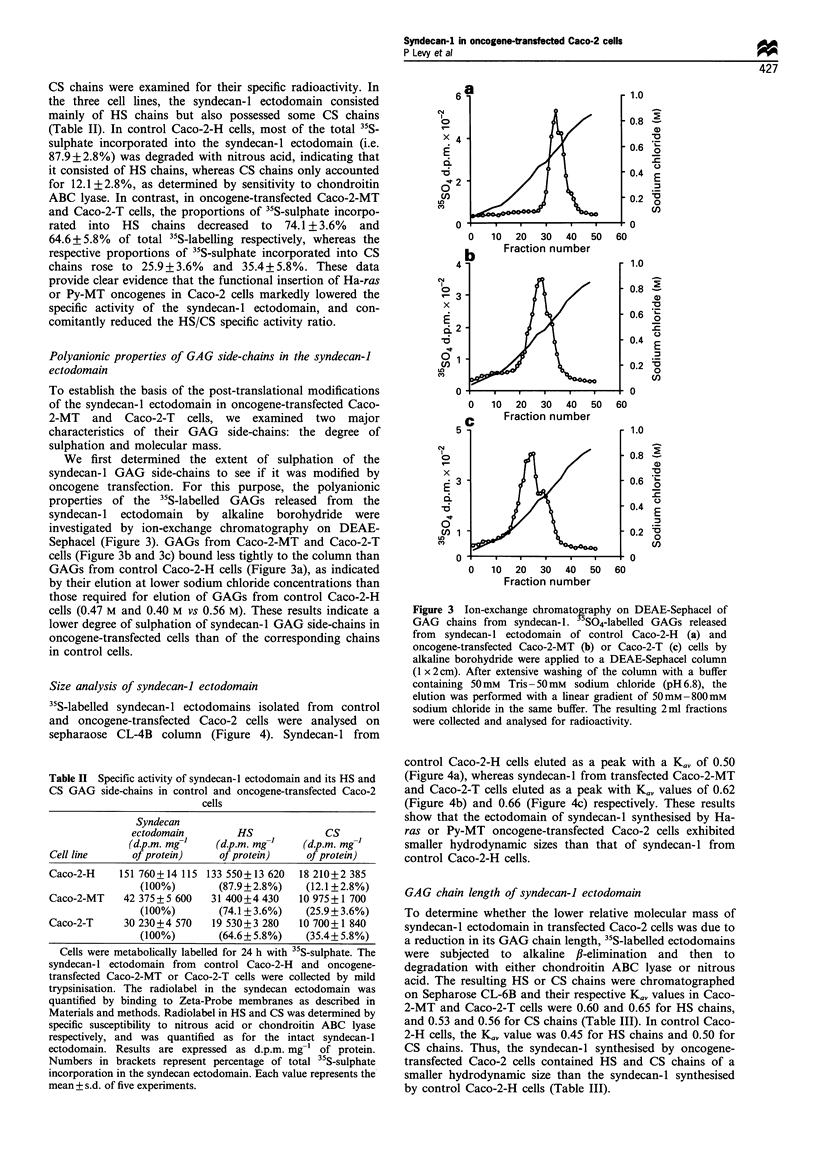
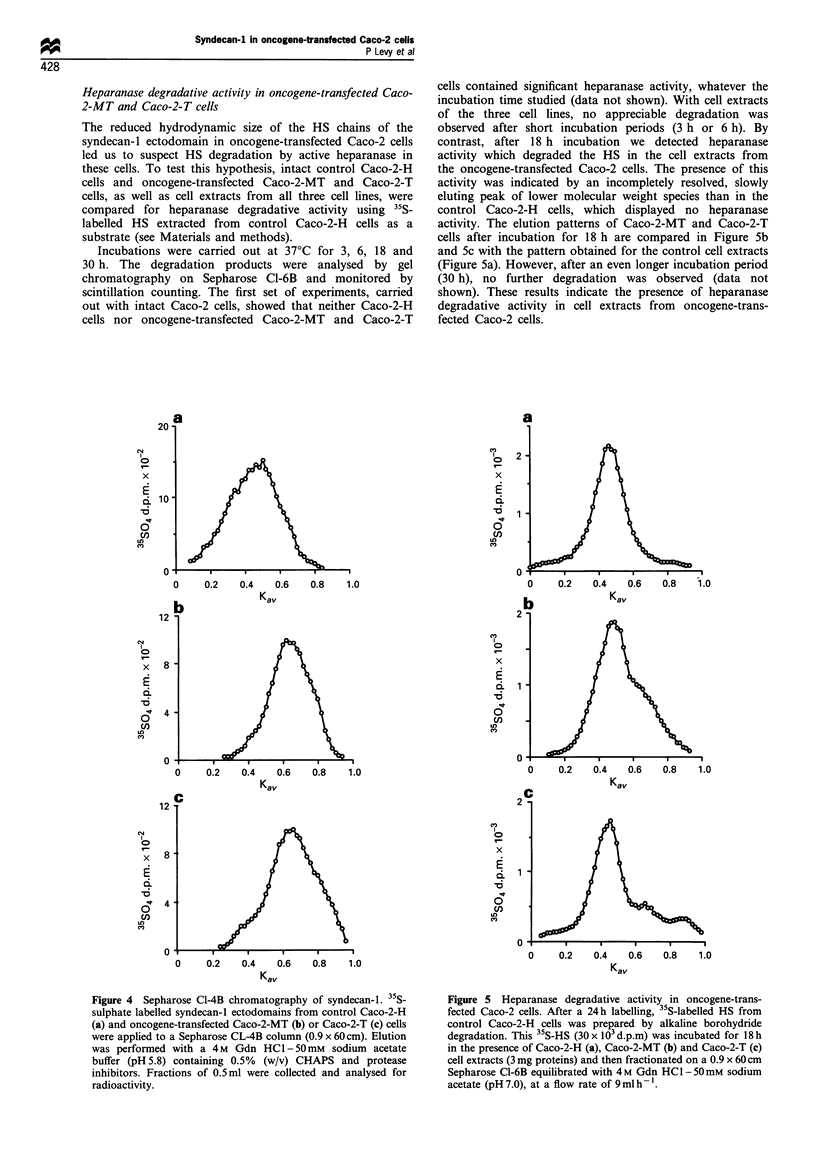
The products of ras and src proto-oncogenes are frequently activated in a constitutive state in human colorectal cancer. In this study we attempted to establish whether the tumorigenic progression induced by oncogenic activation of p21ras and pp60c-src in human colonic Caco-2 cells is associated with specific alterations of syndecan-1, a membrane-anchored proteoglycan playing a role in cell-matrix interaction and neoplastic growth control. To this end, we used Caco-2 cells made highly tumorigenic by transfection with an activated (Val 12) human Ha-ras gene or with the polyoma middle T (Py-MT) oncogene, a constitutive activator of pp60c-src tyrosine kinase activity. Compared with control vector-transfected Caco-2 cells, both oncogene-transfected cell lines (1) contained smaller amounts of membrane-anchored PGs; (2) exhibited decreased syndecan-1 expression at the protein but not the mRNA level; (3) synthesized 35S-labelled syndecan-1 with decreased specific activity; (4) produced a syndecan-1 ectodomain with a lower molecular mass and reduced GAG chain size and sulphation; and (5) expressed heparanase degradative activity. These results show that the dramatic activation of the tumorigenic potential induced by oncogenic p21ras or Py-MT/pp60c-src in Caco-2 cells is associated with marked alterations of syndecan-1 expression at the translational and post-translational levels.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron-Delage S., Capeau J., Barbu V., Chastre E., Levy P., Gespach C., Cherqui G. Reduced insulin receptor expression and function in human colonic Caco-2 cells by ras and polyoma middle T oncogenes. J Biol Chem. 1994 Jul 15;269(28):18686–18693. [PubMed] [Google Scholar]
- Bernfield M., Kokenyesi R., Kato M., Hinkes M. T., Spring J., Gallo R. L., Lose E. J. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Veillette A., Schwartz A. M., DeSeau V., Rosen N. Activation of pp60c-src protein kinase activity in human colon carcinoma. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2251–2255. doi: 10.1073/pnas.84.8.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Buee L., Boyle N. J., Zhang L. B., Delacourte A., Fillit H. M. Optimization of an alcian blue dot-blot assay for the detection of glycosaminoglycans and proteoglycans. Anal Biochem. 1991 Jun;195(2):238–242. doi: 10.1016/0003-2697(91)90323-l. [DOI] [PubMed] [Google Scholar]
- Carey D. J., Stahl R. C., Cizmeci-Smith G., Asundi V. K. Syndecan-1 expressed in Schwann cells causes morphological transformation and cytoskeletal reorganization and associates with actin during cell spreading. J Cell Biol. 1994 Jan;124(1-2):161–170. doi: 10.1083/jcb.124.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright C. A., Meisler A. I., Eckhart W. Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis. Proc Natl Acad Sci U S A. 1990 Jan;87(2):558–562. doi: 10.1073/pnas.87.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastre E., Empereur S., Di Gioia Y., el Mahdani N., Mareel M., Vleminckx K., Van Roy F., Bex V., Emami S., Spandidos D. A. Neoplastic progression of human and rat intestinal cell lines after transfer of the ras and polyoma middle T oncogenes. Gastroenterology. 1993 Dec;105(6):1776–1789. doi: 10.1016/0016-5085(93)91076-t. [DOI] [PubMed] [Google Scholar]
- David G. Integral membrane heparan sulfate proteoglycans. FASEB J. 1993 Aug;7(11):1023–1030. doi: 10.1096/fasebj.7.11.8370471. [DOI] [PubMed] [Google Scholar]
- Delage S., Chastre E., Empereur S., Wicek D., Veissiére D., Capeau J., Gespach C., Cherqui G. Increased protein kinase C alpha expression in human colonic Caco-2 cells after insertion of human Ha-ras or polyoma virus middle T oncogenes. Cancer Res. 1993 Jun 15;53(12):2762–2770. [PubMed] [Google Scholar]
- Elenius K., Vainio S., Laato M., Salmivirta M., Thesleff I., Jalkanen M. Induced expression of syndecan in healing wounds. J Cell Biol. 1991 Aug;114(3):585–595. doi: 10.1083/jcb.114.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimelius B., Norling B., Westermark B., Wasteson A. Composition and distribution of glycosaminoglycans in cultures of human normal and malignant glial cells. Biochem J. 1978 Jun 15;172(3):443–456. doi: 10.1042/bj1720443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inki P., Kujari H., Jalkanen M. Syndecan in carcinomas produced from transformed epithelial cells in nude mice. Lab Invest. 1992 Mar;66(3):314–323. [PubMed] [Google Scholar]
- Inki P., Larjava H., Haapasalmi K., Miettinen H. M., Grenman R., Jalkanen M. Expression of syndecan-1 is induced by differentiation and suppressed by malignant transformation of human keratinocytes. Eur J Cell Biol. 1994 Feb;63(1):43–51. [PubMed] [Google Scholar]
- Inki P., Stenbäck F., Talve L., Jalkanen M. Immunohistochemical localization of syndecan in mouse skin tumors induced by UV irradiation. Loss of expression associated with malignant transformation. Am J Pathol. 1991 Dec;139(6):1333–1340. [PMC free article] [PubMed] [Google Scholar]
- Iozzo R. V. Proteoglycans and neoplasia. Cancer Metastasis Rev. 1988 Apr;7(1):39–50. doi: 10.1007/BF00048277. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V. Proteoglycans: structure, function, and role in neoplasia. Lab Invest. 1985 Oct;53(4):373–396. [PubMed] [Google Scholar]
- Iozzo R. V., Sampson P. M., Schmitt G. K. Neoplastic modulation of extracellular matrix: stimulation of chondroitin sulfate proteoglycan and hyaluronic acid synthesis in co-cultures of human colon carcinoma and smooth muscle cells. J Cell Biochem. 1989 Apr;39(4):355–378. doi: 10.1002/jcb.240390403. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V., Wight T. N. Isolation and characterization of proteoglycans synthesized by human colon and colon carcinoma. J Biol Chem. 1982 Sep 25;257(18):11135–11144. [PubMed] [Google Scholar]
- Jalkanen M., Nguyen H., Rapraeger A., Kurn N., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells: localization on the cell surface with a monoclonal antibody. J Cell Biol. 1985 Sep;101(3):976–984. doi: 10.1083/jcb.101.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen M., Rapraeger A., Saunders S., Bernfield M. Cell surface proteoglycan of mouse mammary epithelial cells is shed by cleavage of its matrix-binding ectodomain from its membrane-associated domain. J Cell Biol. 1987 Dec;105(6 Pt 2):3087–3096. doi: 10.1083/jcb.105.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirjavainen J., Leppä S., Hynes N. E., Jalkanen M. Translational suppression of syndecan-1 expression in Ha-ras transformed mouse mammary epithelial cells. Mol Biol Cell. 1993 Aug;4(8):849–858. doi: 10.1091/mbc.4.8.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima J., Nakamura N., Kanatani M., Omori K. The glycosaminoglycans in human hepatic cancer. Cancer Res. 1975 Mar;35(3):542–547. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leppä S., Härkönen P., Jalkanen M. Steroid-induced epithelial-fibroblastic conversion associated with syndecan suppression in S115 mouse mammary tumor cells. Cell Regul. 1991 Jan;2(1):1–11. doi: 10.1091/mbc.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppä S., Mali M., Miettinen H. M., Jalkanen M. Syndecan expression regulates cell morphology and growth of mouse mammary epithelial tumor cells. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):932–936. doi: 10.1073/pnas.89.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy P., Cherqui G., Robert A., Wicek D., Picard J. Changes in glycosaminoglycan sulfation and protein kinase C subcellular distribution during differentiation of the human colon tumor cell line Caco-2. Experientia. 1989 Jun 15;45(6):588–591. doi: 10.1007/BF01990515. [DOI] [PubMed] [Google Scholar]
- Levy P., Loreal O., Munier A., Yamada Y., Picard J., Cherqui G., Clement B., Capeau J. Enterocytic differentiation of the human Caco-2 cell line is correlated with down-regulation of fibronectin and laminin. FEBS Lett. 1994 Feb 7;338(3):272–276. doi: 10.1016/0014-5793(94)80282-3. [DOI] [PubMed] [Google Scholar]
- Levy P., Robert A., Picard J. Biosynthesis of glycosaminoglycans in the human colonic tumor cell line Caco-2: structural changes occurring with the morphological differentiation of the cells. Biol Cell. 1988;62(3):255–264. [PubMed] [Google Scholar]
- Liebersbach B. F., Sanderson R. D. Expression of syndecan-1 inhibits cell invasion into type I collagen. J Biol Chem. 1994 Aug 5;269(31):20013–20019. [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Lévy P., Emami S., Cherqui G., Chastre E., Gespach C., Picard J. Altered expression of proteoglycans in E1A-immortalized rat fetal intestinal epithelial cells in culture. Cancer Res. 1990 Oct 15;50(20):6716–6722. [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Nakajima M., Irimura T., Di Ferrante N., Nicolson G. L. Metastatic melanoma cell heparanase. Characterization of heparan sulfate degradation fragments produced by B16 melanoma endoglucuronidase. J Biol Chem. 1984 Feb 25;259(4):2283–2290. [PubMed] [Google Scholar]
- Pickett C. A., Gutierrez-Hartmann A. Ras mediates Src but not epidermal growth factor-receptor tyrosine kinase signaling pathways in GH4 neuroendocrine cells. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8612–8616. doi: 10.1073/pnas.91.18.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A., Bernfield M. Cell surface proteoglycan of mammary epithelial cells. Protease releases a heparan sulfate-rich ectodomain from a putative membrane-anchored domain. J Biol Chem. 1985 Apr 10;260(7):4103–4109. [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Bernfield M. Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2683–2696. doi: 10.1083/jcb.103.6.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricoveri W., Cappelletti R. Heparan sulfate endoglycosidase and metastatic potential in murine fibrosarcoma and melanoma. Cancer Res. 1986 Aug;46(8):3855–3861. [PubMed] [Google Scholar]
- Rousset M., Dussaulx E., Chevalier G., Zweibaum A. Growth-related glycogen levels of human intestine carcinoma cell lines grown in vitro and in vivo in nude mice. J Natl Cancer Inst. 1980 Nov;65(5):885–889. [PubMed] [Google Scholar]
- Sanderson R. D., Hinkes M. T., Bernfield M. Syndecan-1, a cell-surface proteoglycan, changes in size and abundance when keratinocytes stratify. J Invest Dermatol. 1992 Oct;99(4):390–396. doi: 10.1111/1523-1747.ep12616103. [DOI] [PubMed] [Google Scholar]
- Sanderson R. D., Lalor P., Bernfield M. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1989 Nov;1(1):27–35. doi: 10.1091/mbc.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson R. D., Sneed T. B., Young L. A., Sullivan G. L., Lander A. D. Adhesion of B lymphoid (MPC-11) cells to type I collagen is mediated by integral membrane proteoglycan, syndecan. J Immunol. 1992 Jun 15;148(12):3902–3911. [PubMed] [Google Scholar]
- Sanderson R. D., Turnbull J. E., Gallagher J. T., Lander A. D. Fine structure of heparan sulfate regulates syndecan-1 function and cell behavior. J Biol Chem. 1994 May 6;269(18):13100–13106. [PubMed] [Google Scholar]
- Saunders S., Bernfield M. Cell surface proteoglycan binds mouse mammary epithelial cells to fibronectin and behaves as a receptor for interstitial matrix. J Cell Biol. 1988 Feb;106(2):423–430. doi: 10.1083/jcb.106.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S., Jalkanen M., O'Farrell S., Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989 Apr;108(4):1547–1556. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Liotta L. A., Kleiner D. E., Jr Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993 Dec;7(15):1434–1441. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- Trainer D. L., Kline T., McCabe F. L., Faucette L. F., Feild J., Chaikin M., Anzano M., Rieman D., Hoffstein S., Li D. J. Biological characterization and oncogene expression in human colorectal carcinoma cell lines. Int J Cancer. 1988 Feb 15;41(2):287–296. doi: 10.1002/ijc.2910410221. [DOI] [PubMed] [Google Scholar]
- Vainio S., Jalkanen M., Bernfield M., Saxén L. Transient expression of syndecan in mesenchymal cell aggregates of the embryonic kidney. Dev Biol. 1992 Aug;152(2):221–232. doi: 10.1016/0012-1606(92)90130-9. [DOI] [PubMed] [Google Scholar]
- Vainio S., Jalkanen M., Vaahtokari A., Sahlberg C., Mali M., Bernfield M., Thesleff I. Expression of syndecan gene is induced early, is transient, and correlates with changes in mesenchymal cell proliferation during tooth organogenesis. Dev Biol. 1991 Oct;147(2):322–333. doi: 10.1016/0012-1606(91)90290-j. [DOI] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Winterbourne D. J., Mora P. T. Altered metabolism of heparan sulfate in simian virus 40 transformed cloned mouse cells. J Biol Chem. 1978 Jul 25;253(14):5109–5120. [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Proteoglycans synthesized by rat ovarian granulosa cells in culture. Isolation, fractionation, and characterization of proteoglycans associated with the cell layer. J Biol Chem. 1984 Aug 25;259(16):10260–10269. [PubMed] [Google Scholar]
- Yeaman C., Rapraeger A. C. Membrane-anchored proteoglycans of mouse macrophages: P388D1 cells express a syndecan-4-like heparan sulfate proteoglycan and a distinct chondroitin sulfate form. J Cell Physiol. 1993 Nov;157(2):413–425. doi: 10.1002/jcp.1041570226. [DOI] [PubMed] [Google Scholar]