Abstract
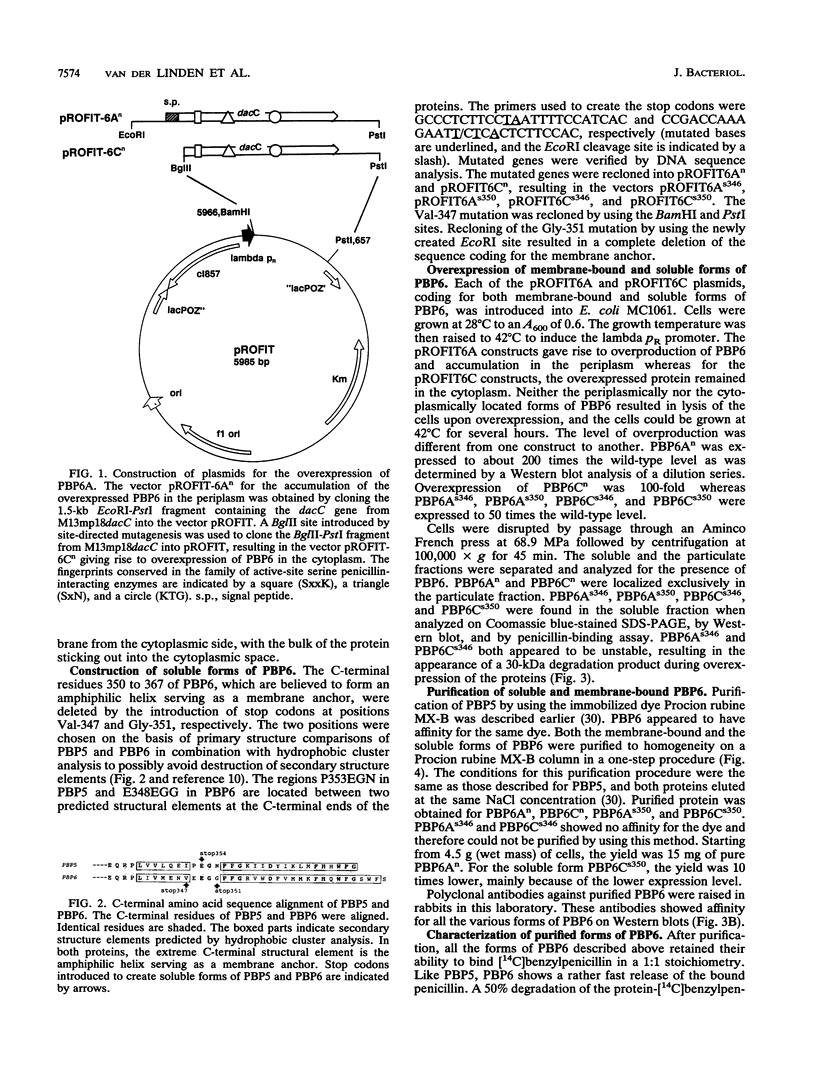
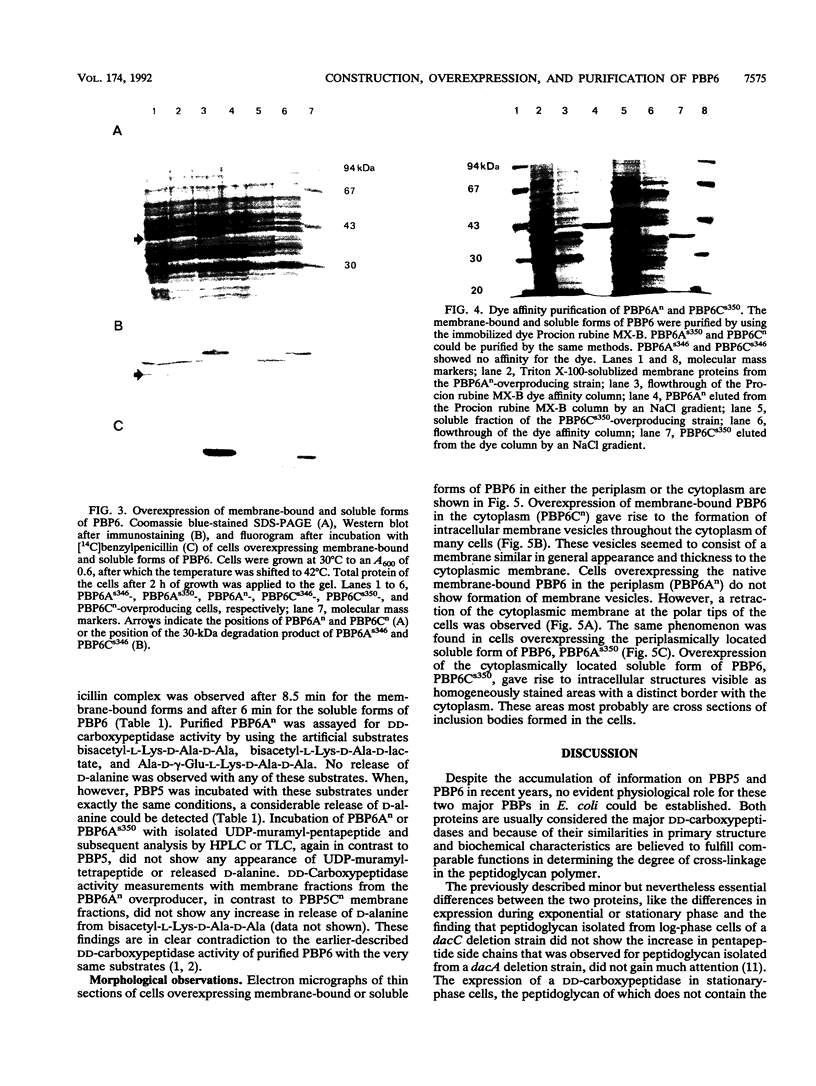
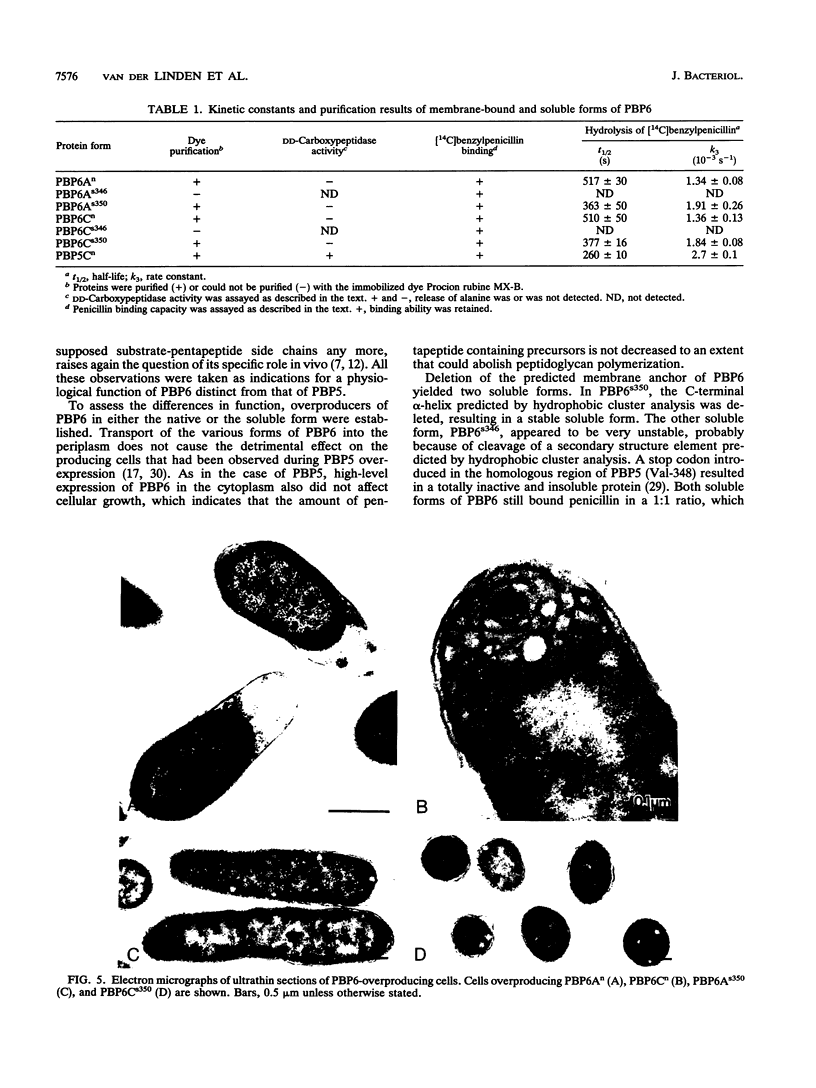
Plasmids for high-level expression of penicillin-binding protein 6 (PBP6) were constructed, giving rise to overproduction of PBP6 under the control of the lambda pR promoter in either the periplasmic or the cytoplasmic space. In contrast to penicillin-binding protein 5 (PBP5), the presence of high amounts of PBP6 in the periplasm as well as in the cytoplasm did not result in growth as spherical cells or in lysis. Deletion of the C-terminal membrane anchor of PBP6 resulted in a soluble form of the protein (PBP6s350). Electron micrographs of thin sections of cells overexpressing both native membrane-bound and soluble PBP6 in the periplasm revealed a polar retraction of the cytoplasmic membrane. Cytoplasmic overexpression of native PBP6 gave rise to the formation of membrane vesicles, whereas the soluble PBP6 formed inclusion bodies in the cytoplasm. Both the membrane-bound and the soluble forms of PBP6 were purified to homogeneity by using the immobilized dye Procion rubine MX-B. Purified preparations of PBP6 and PBP6s350 formed a 14[C]penicillin-protein complex at a 1:1 stoichiometry. The half-lives of the complexes were 8.5 and 6 min, respectively. In contrast to PBP5, no DD-carboxypeptidase activity could be detected for PBP6 by using bisacetyl-L-Lys-D-Ala-D-Ala and several other substrates. These findings led us to conclude that PBP6 has a biological function clearly distinct from that of PBP5 and to suggest a role for PBP6 in the stabilization of the peptidoglycan during stationary phase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amanuma H., Strominger J. L. Purification and properties of penicillin-binding proteins 5 and 6 from Escherichia coli membranes. J Biol Chem. 1980 Dec 10;255(23):11173–11180. [PubMed] [Google Scholar]
- Amanuma H., Strominger J. L. Purification and properties of penicillin-binding proteins 5 and 6 from the dacA mutant strain of Escherichia coli (JE 11191). J Biol Chem. 1984 Jan 25;259(2):1294–1298. [PubMed] [Google Scholar]
- Bittner M., Kupferer P., Morris C. F. Electrophoretic transfer of proteins and nucleic acids from slab gels to diazobenzyloxymethyl cellulose or nitrocellulose sheets. Anal Biochem. 1980 Mar 1;102(2):459–471. doi: 10.1016/0003-2697(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broome-Smith J. K., Ioannidis I., Edelman A., Spratt B. G. Nucleotide sequences of the penicillin-binding protein 5 and 6 genes of Escherichia coli. Nucleic Acids Res. 1988 Feb 25;16(4):1617–1617. doi: 10.1093/nar/16.4.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Ling M. L. Isolation and sequence analysis of dacB, which encodes a sporulation-specific penicillin-binding protein in Bacillus subtilis. J Bacteriol. 1992 Mar;174(6):1717–1725. doi: 10.1128/jb.174.6.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C. E., Sowell M. O. Synthesis of penicillin-binding protein 6 by stationary-phase Escherichia coli. J Bacteriol. 1982 Jul;151(1):491–494. doi: 10.1128/jb.151.1.491-494.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira L. C., Schwarz U., Keck W., Charlier P., Dideberg O., Ghuysen J. M. Properties and crystallization of a genetically engineered, water-soluble derivative of penicillin-binding protein 5 of Escherichia coli K12. Eur J Biochem. 1988 Jan 15;171(1-2):11–16. doi: 10.1111/j.1432-1033.1988.tb13751.x. [DOI] [PubMed] [Google Scholar]
- Frére J. M., Leyh-Bouille M., Ghuysen J. M., Nieto M., Perkins H. R. Exocellular DD-carboxypeptidases-transpeptidases from Streptomyces. Methods Enzymol. 1976;45:610–636. doi: 10.1016/s0076-6879(76)45054-1. [DOI] [PubMed] [Google Scholar]
- Gaboriaud C., Bissery V., Benchetrit T., Mornon J. P. Hydrophobic cluster analysis: an efficient new way to compare and analyse amino acid sequences. FEBS Lett. 1987 Nov 16;224(1):149–155. doi: 10.1016/0014-5793(87)80439-8. [DOI] [PubMed] [Google Scholar]
- Glauner B., Höltje J. V. Growth pattern of the murein sacculus of Escherichia coli. J Biol Chem. 1990 Nov 5;265(31):18988–18996. [PubMed] [Google Scholar]
- Goodell W., Tomasz A. Alteration of Escherichia coli murein during amino acid starvation. J Bacteriol. 1980 Dec;144(3):1009–1016. doi: 10.1128/jb.144.3.1009-1016.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. E., Pratt J. M. An 18 amino acid amphiphilic helix forms the membrane-anchoring domain of the Escherichia coli penicillin-binding protein 5. Mol Microbiol. 1987 Jul;1(1):23–28. doi: 10.1111/j.1365-2958.1987.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Markiewicz Z., Broome-Smith J. K., Schwarz U., Spratt B. G. Spherical E. coli due to elevated levels of D-alanine carboxypeptidase. Nature. 1982 Jun 24;297(5868):702–704. doi: 10.1038/297702a0. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Tamaki S., Curtis S. J., Strominger J. L. Mutational evidence for identity of penicillin-binding protein 5 in Escherichia coli with the major D-alanine carboxypeptidase IA activity. J Bacteriol. 1979 Jan;137(1):644–647. doi: 10.1128/jb.137.1.644-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner P. S., Sisk W. P., Berman M. L. Bacteriophage lambda cloning system for the construction of directional cDNA libraries. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4171–4175. doi: 10.1073/pnas.84.12.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Pratt J. M., Holland I. B., Spratt B. G. Precursor forms of penicillin-binding proteins 5 and 6 of E. coli cytoplasmic membrane. Nature. 1981 Sep 24;293(5830):307–309. doi: 10.1038/293307a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Strominger J. L. Identification of the major penicillin-binding proteins of Escherichia coli as D-alanine carboxypeptidase IA. J Bacteriol. 1976 Jul;127(1):660–663. doi: 10.1128/jb.127.1.660-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P. J., Knobler R. L., Buchmeier M. J. Western and dot immunoblotting analysis of viral antigens and antibodies: application to murine hepatitis virus. J Immunol Methods. 1984 Oct 12;73(1):177–188. doi: 10.1016/0022-1759(84)90043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- Todd J. A., Bone E. J., Ellar D. J. The sporulation-specific penicillin-binding protein 5a from Bacillus subtilis is a DD-carboxypeptidase in vitro. Biochem J. 1985 Sep 15;230(3):825–828. doi: 10.1042/bj2300825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. H., Lemire B. D., Elmes M. L., Bradley R. D., Scraba D. G. Overproduction of fumarate reductase in Escherichia coli induces a novel intracellular lipid-protein organelle. J Bacteriol. 1984 May;158(2):590–596. doi: 10.1128/jb.158.2.590-596.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden M. P., Mottl H., Keck W. Cytoplasmic high-level expression of a soluble, enzymatically active form of the Escherichia coli penicillin-binding protein 5 and purification by dye chromatography. Eur J Biochem. 1992 Feb 15;204(1):197–202. doi: 10.1111/j.1432-1033.1992.tb16624.x. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., van Deurs B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984 Aug;3(8):1791–1797. doi: 10.1002/j.1460-2075.1984.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]