Abstract
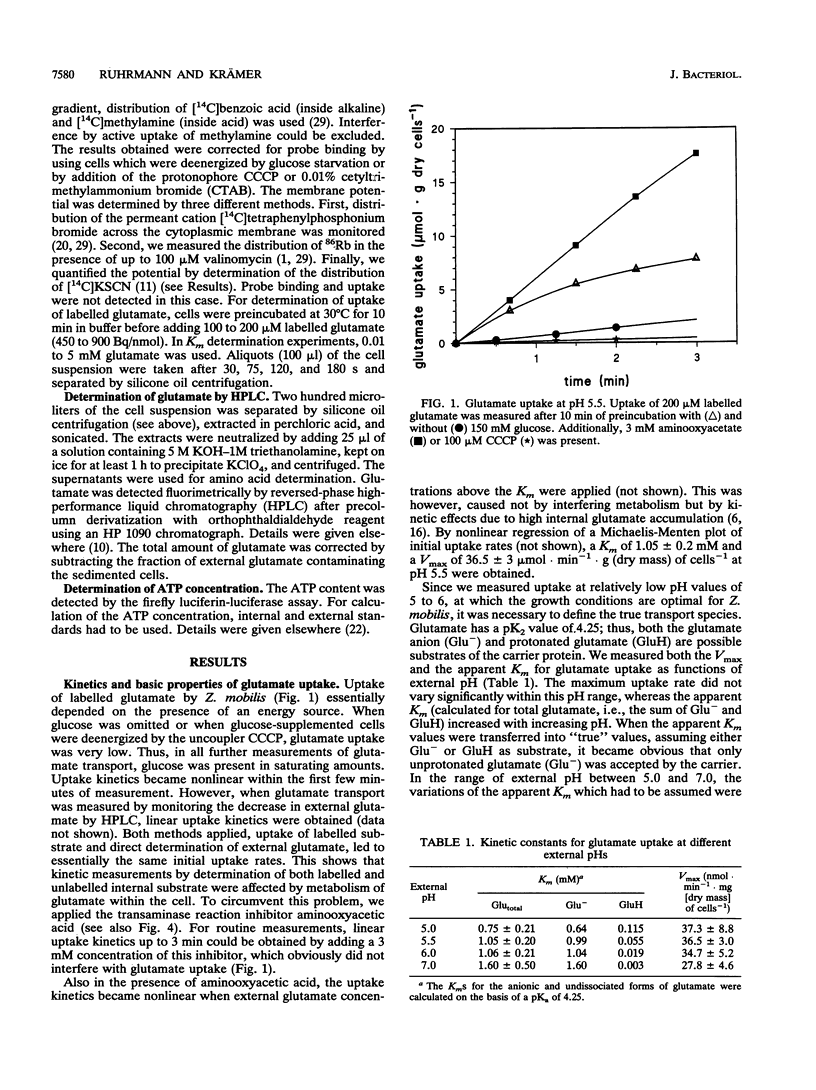
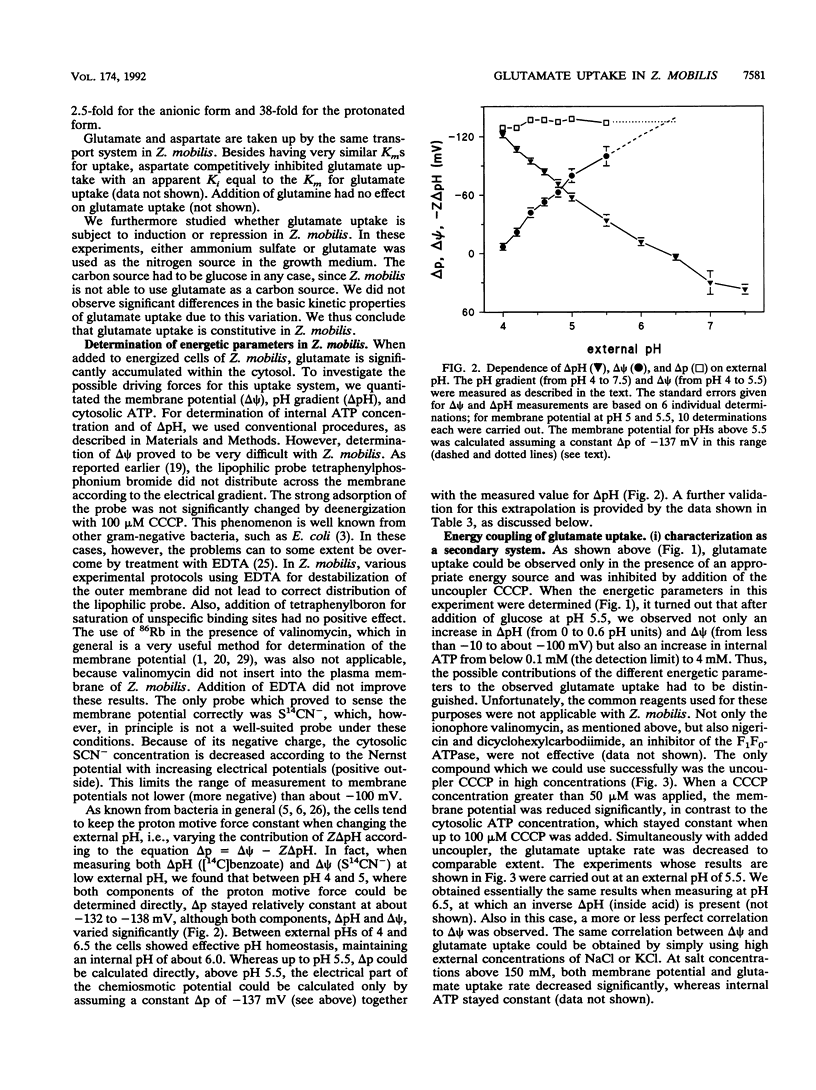
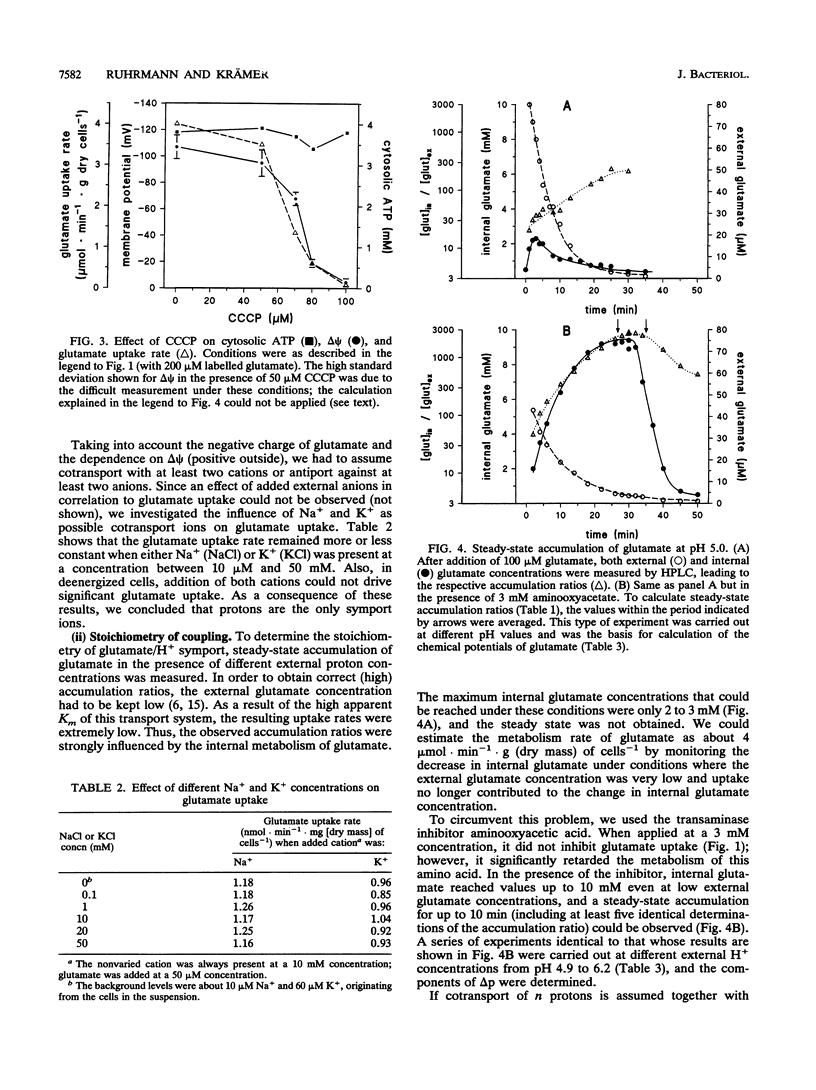
The energetics of the anaerobic gram-negative bacterium Zymomonas mobilis, a well-known ethanol-producing organism, is based solely on synthesis of 1 mol of ATP per mol of glucose by the Entner-Doudoroff pathway. When grown in the presence of glucose as a carbon and energy source, Z. mobilis had a cytosolic ATP content of 3.5 to 4 mM. Because of effective pH homeostasis, the components of the proton motive force strongly depended on the external pH. At pH 5.5, i.e., around the optimal pH for growth, the proton motive force was about -135 mV and was composed of a pH gradient of 0.6 pH units (internal pH 6.1) and a membrane potential of about -100 mV. Measurement of these parameters was complicated since ionophores and lipophilic probes were ineffective in this organism. So far, only glucose transport by facilitated diffusion is well characterized for Z. mobilis. We investigated a constitutive secondary glutamate uptake system. Glutamate can be used as a nitrogen source for Z. mobilis. Transport of glutamate at pH 5.5 shows a relatively high Vmax of 40 mumol.min-1.g (dry mass) of cells-1 and a low affinity (Km = 1.05 mM). Glutamate is taken up by a symport with two H+ ions, leading to substantial accumulation in the cytosol at low pH values.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S., Booth I. R. The use of valinomycin, nigericin and trichlorocarbanilide in control of the protonmotive force in Escherichia coli cells. Biochem J. 1983 Apr 15;212(1):105–112. doi: 10.1042/bj2120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnell W. O., Yi K. C., Conway T. Sequence and genetic organization of a Zymomonas mobilis gene cluster that encodes several enzymes of glucose metabolism. J Bacteriol. 1990 Dec;172(12):7227–7240. doi: 10.1128/jb.172.12.7227-7240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S., Krämer R. Lysine uptake and exchange in Corynebacterium glutamicum. J Bacteriol. 1990 Dec;172(12):7241–7248. doi: 10.1128/jb.172.12.7241-7248.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. C., Nicholls D. G., Ingledew W. J. Transmembrane electrical potential and transmembrane pH gradient in the acidophile Thiobacillus ferro-oxidans. Biochem J. 1979 Jan 15;178(1):195–200. doi: 10.1042/bj1780195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi Y., Yamato I., Anraku Y. Nucleotide sequence of gltS, the Na+/glutamate symport carrier gene of Escherichia coli B. J Biol Chem. 1990 Dec 15;265(35):21704–21708. [PubMed] [Google Scholar]
- Dimarco A. A., Romano A. H. d-Glucose Transport System of Zymomonas mobilis. Appl Environ Microbiol. 1985 Jan;49(1):151–157. doi: 10.1128/aem.49.1.151-157.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Hellingwerf K. J., Konings W. N. Mechanism of energy coupling to entry and exit of neutral and branched chain amino acids in membrane vesicles of Streptococcus cremoris. J Biol Chem. 1987 Sep 15;262(26):12438–12443. [PubMed] [Google Scholar]
- Fujimura T., Yamato I., Anraku Y. Mechanism of glutamate transport in Escherichia coli B. 1. Proton-dependent and sodium ion dependent binding of glutamate to a glutamate carrier in the cytoplasmic membrane. Biochemistry. 1983 Apr 12;22(8):1954–1959. doi: 10.1021/bi00277a033. [DOI] [PubMed] [Google Scholar]
- Heller K. B., Lin E. C., Wilson T. H. Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol. 1980 Oct;144(1):274–278. doi: 10.1128/jb.144.1.274-278.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- Krämer R., Lambert C. Uptake of glutamate in Corynebacterium glutamicum. 2. Evidence for a primary active transport system. Eur J Biochem. 1990 Dec 27;194(3):937–944. doi: 10.1111/j.1432-1033.1990.tb19489.x. [DOI] [PubMed] [Google Scholar]
- Padan E., Rottenberg H. Respiratory control and the proton electrochemical gradient in mitochondria. Eur J Biochem. 1973 Dec 17;40(2):431–437. doi: 10.1111/j.1432-1033.1973.tb03212.x. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Konings W. N. Kinetic properties of a phosphate-bond-driven glutamate-glutamine transport system in Streptococcus lactis and Streptococcus cremoris. J Bacteriol. 1987 Jun;169(6):2755–2761. doi: 10.1128/jb.169.6.2755-2761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Russell J. B. Low-affinity, high-capacity system of glucose transport in the ruminal bacterium Streptococcus bovis: evidence for a mechanism of facilitated diffusion. Appl Environ Microbiol. 1990 Nov;56(11):3304–3307. doi: 10.1128/aem.56.11.3304-3307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg G. D., Furlong C. E. Resolution of the multiplicity of the glutamate and aspartate transport systems of Escherichia coli. J Biol Chem. 1977 Dec 25;252(24):9055–9064. [PubMed] [Google Scholar]
- Swings J., De Ley J. The biology of Zymomonas. Bacteriol Rev. 1977 Mar;41(1):1–46. doi: 10.1128/br.41.1.1-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace B., Yang Y. J., Hong J. S., Lum D. Cloning and sequencing of a gene encoding a glutamate and aspartate carrier of Escherichia coli K-12. J Bacteriol. 1990 Jun;172(6):3214–3220. doi: 10.1128/jb.172.6.3214-3220.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij W., Bulthuis R. A., van Iwaarden P. R., Konings W. N. Mechanism of L-glutamate transport in membrane vesicles from Bacillus stearothermophilus. J Bacteriol. 1989 Feb;171(2):1118–1125. doi: 10.1128/jb.171.2.1118-1125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


