Abstract
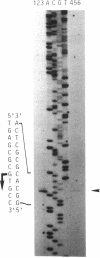
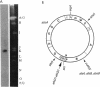
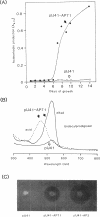
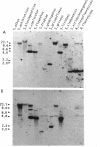
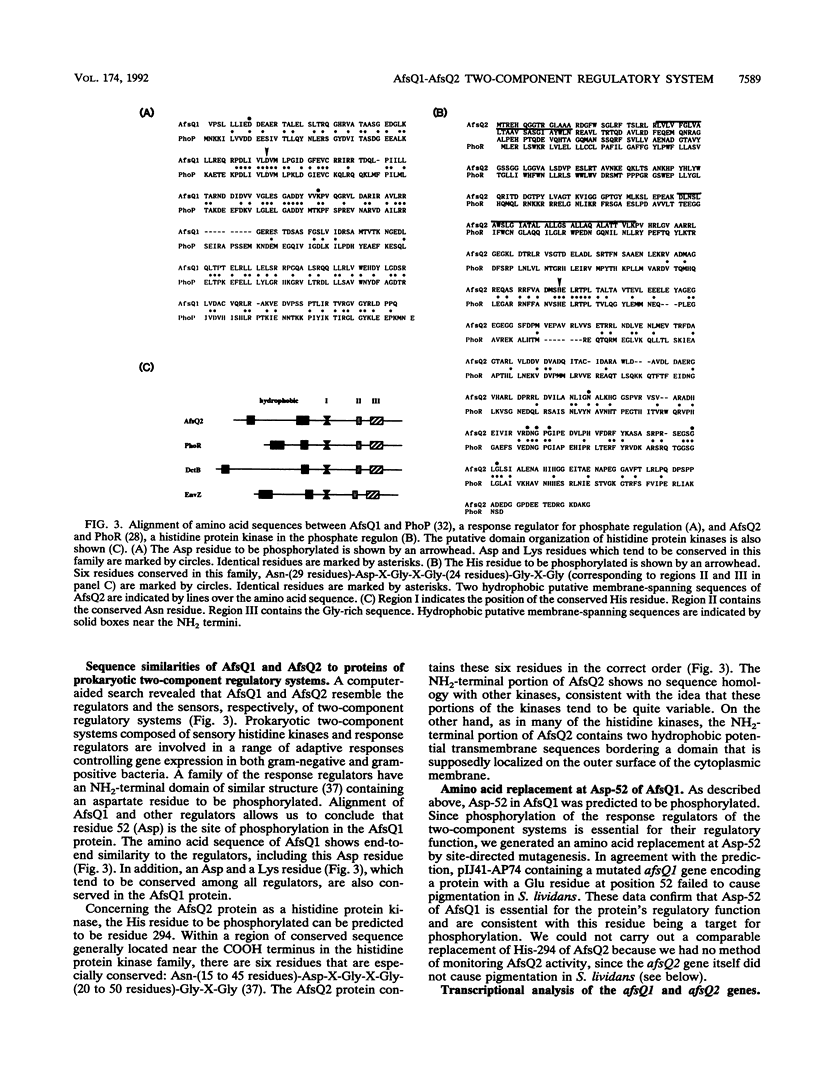
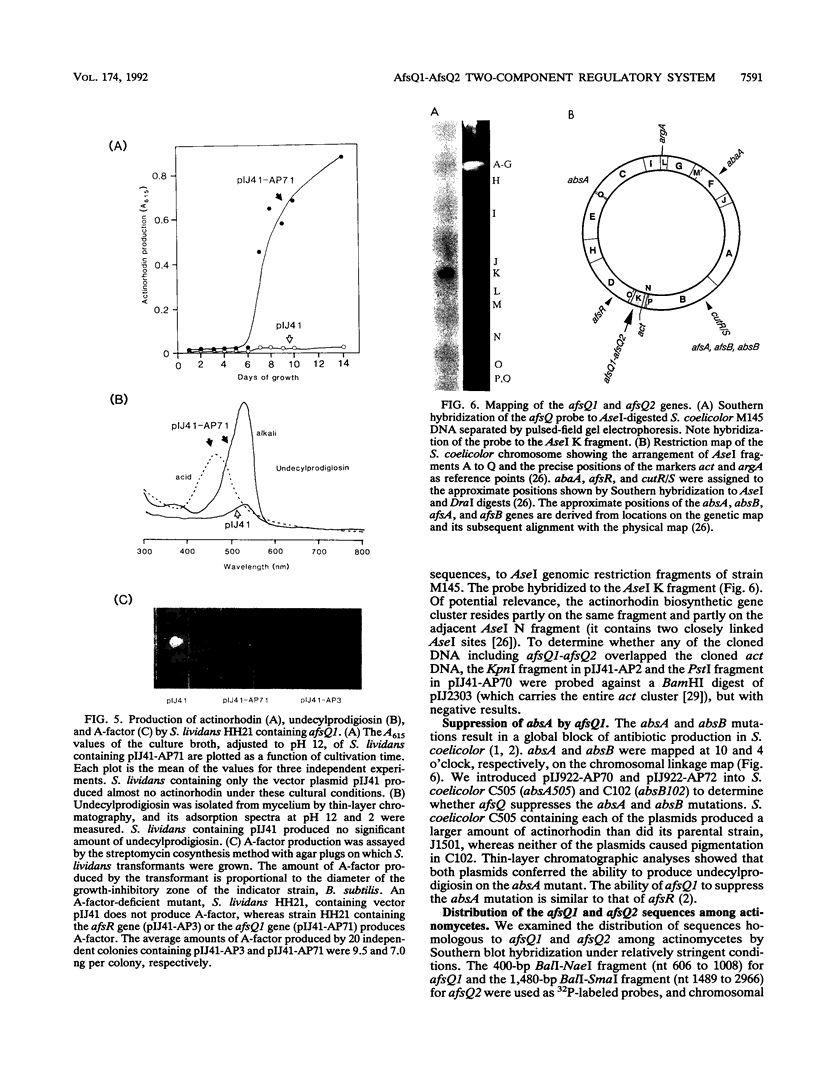
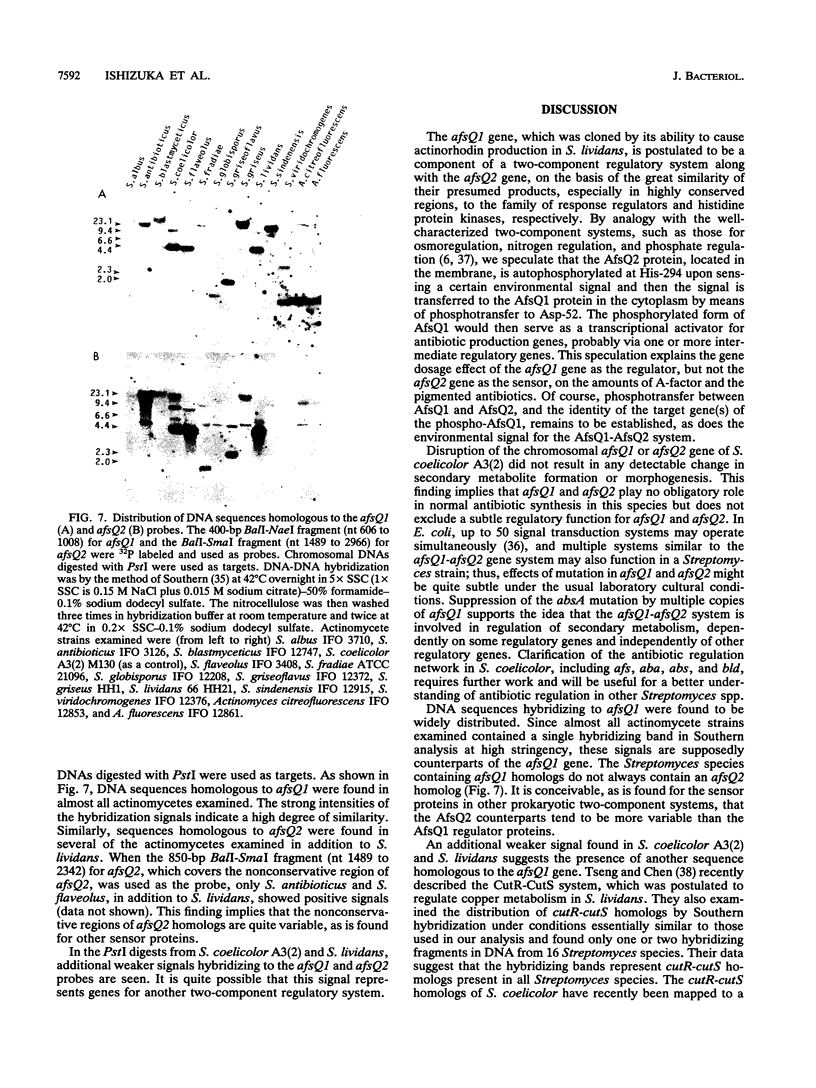
A DNA fragment stimulating actinorhodin, undecylprodigiosin, and A-factor production in Streptomyces lividans 66 was cloned from Streptomyces coelicolor A3(2). Nucleotide sequencing revealed the presence of an open reading frame of 225 codons, named afsQ1, that showed great similarity in amino acid sequence to the response regulators of typical prokaryotic two-component regulatory systems responsible for adaptive responses. The termination codon, TGA, of afsQ1 overlapped the initiation codon, GTG, of a second open reading frame, afsQ2, of 535 codons. The afsQ2 gene product showed homology with the sensory histidine protein kinases of two-component systems. In agreement with the assumption that the AfsQ1 and AfsQ2 proteins comprise an aspartate-histidine phosphotransfer system, an amino acid replacement from Asp to Glu at residue 52 of AfsQ1, generated by site-directed mutagenesis, resulted in loss of the protein's ability to stimulate antibiotic production in S. lividans. Primer extension experiments indicated that transcription of the afsQ1 and afsQ2 genes initiates at the translational start codon (GTG) of the afsQ1 gene. The afsQ1 and afsQ2 genes were physically mapped at a chromosomal position near the actinorhodin biosynthetic gene cluster (act) by hybridization to Southern blots of restriction fragments separated by pulsed-field gel electrophoresis. Disruption of either afsQ1 or afsQ2 on the S. coelicolor chromosome by use of phage phi C31KC515 led to no detectable change in secondary metabolite formation or morphogenesis. The afsQ1 gene on pIJ922 suppressed the S. coelicolor absA mutation and caused actinorhodin production but did not suppress the absB mutation. Southern blot hybridization showed that sequences homologous to afsQ1 and afsQ2 are present in almost all of the actinomycetes examined.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamidis T., Champness W. Genetic analysis of absB, a Streptomyces coelicolor locus involved in global antibiotic regulation. J Bacteriol. 1992 Jul;174(14):4622–4628. doi: 10.1128/jb.174.14.4622-4628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamidis T., Riggle P., Champness W. Mutations in a new Streptomyces coelicolor locus which globally block antibiotic biosynthesis but not sporulation. J Bacteriol. 1990 Jun;172(6):2962–2969. doi: 10.1128/jb.172.6.2962-2969.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Bourret R. B., Borkovich K. A., Simon M. I. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu Rev Biochem. 1991;60:401–441. doi: 10.1146/annurev.bi.60.070191.002153. [DOI] [PubMed] [Google Scholar]
- Fernández-Moreno M. A., Caballero J. L., Hopwood D. A., Malpartida F. The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA gene of Streptomyces. Cell. 1991 Aug 23;66(4):769–780. doi: 10.1016/0092-8674(91)90120-n. [DOI] [PubMed] [Google Scholar]
- Fernández-Moreno M. A., Martín-Triana A. J., Martínez E., Niemi J., Kieser H. M., Hopwood D. A., Malpartida F. abaA, a new pleiotropic regulatory locus for antibiotic production in Streptomyces coelicolor. J Bacteriol. 1992 May;174(9):2958–2967. doi: 10.1128/jb.174.9.2958-2967.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. H., Wray L. V., Jr Regulation of glutamine synthetase in Streptomyces coelicolor. J Bacteriol. 1989 May;171(5):2378–2383. doi: 10.1128/jb.171.5.2378-2383.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara O., Beppu T. Mutants blocked in streptomycin production in Streptomyces griseus - the role of A-factor. J Antibiot (Tokyo) 1982 Mar;35(3):349–358. doi: 10.7164/antibiotics.35.349. [DOI] [PubMed] [Google Scholar]
- Hong S. K., Kito M., Beppu T., Horinouchi S. Phosphorylation of the AfsR product, a global regulatory protein for secondary-metabolite formation in Streptomyces coelicolor A3(2). J Bacteriol. 1991 Apr;173(7):2311–2318. doi: 10.1128/jb.173.7.2311-2318.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D. A., Bibb M. J., Chater K. F., Kieser T. Plasmid and phage vectors for gene cloning and analysis in Streptomyces. Methods Enzymol. 1987;153:116–166. doi: 10.1016/0076-6879(87)53052-x. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A. The Leeuwenhoek lecture, 1987. Towards an understanding of gene switching in Streptomyces, the basis of sporulation and antibiotic production. Proc R Soc Lond B Biol Sci. 1988 Nov 22;235(1279):121–138. doi: 10.1098/rspb.1988.0067. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Furuya K., Nishiyama M., Suzuki H., Beppu T. Nucleotide sequence of the streptothricin acetyltransferase gene from Streptomyces lavendulae and its expression in heterologous hosts. J Bacteriol. 1987 May;169(5):1929–1937. doi: 10.1128/jb.169.5.1929-1937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Hara O., Beppu T. Cloning of a pleiotropic gene that positively controls biosynthesis of A-factor, actinorhodin, and prodigiosin in Streptomyces coelicolor A3(2) and Streptomyces lividans. J Bacteriol. 1983 Sep;155(3):1238–1248. doi: 10.1128/jb.155.3.1238-1248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Ishizuka H., Beppu T. Cloning, nucleotide sequence, and transcriptional analysis of the NAD(P)-dependent cholesterol dehydrogenase gene from a Nocardia sp. and its hyperexpression in Streptomyces spp. Appl Environ Microbiol. 1991 May;57(5):1386–1393. doi: 10.1128/aem.57.5.1386-1393.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Kito M., Nishiyama M., Furuya K., Hong S. K., Miyake K., Beppu T. Primary structure of AfsR, a global regulatory protein for secondary metabolite formation in Streptomyces coelicolor A3(2). Gene. 1990 Oct 30;95(1):49–56. doi: 10.1016/0378-1119(90)90412-k. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Kumada Y., Beppu T. Unstable genetic determinant of A-factor biosynthesis in streptomycin-producing organisms: cloning and characterization. J Bacteriol. 1984 May;158(2):481–487. doi: 10.1128/jb.158.2.481-487.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Suzuki H., Nishiyama M., Beppu T. Nucleotide sequence and transcriptional analysis of the Streptomyces griseus gene (afsA) responsible for A-factor biosynthesis. J Bacteriol. 1989 Feb;171(2):1206–1210. doi: 10.1128/jb.171.2.1206-1210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen G. R., Ward J. M., Bibb M. J. Unusual transcriptional and translational features of the aminoglycoside phosphotransferase gene (aph) from Streptomyces fradiae. Genes Dev. 1989 Mar;3(3):415–429. doi: 10.1101/gad.3.3.415. [DOI] [PubMed] [Google Scholar]
- Khokhlov A. S., Tovarova I. I., Borisova L. N., Pliner S. A., Shevchenko L. N., Kornitskaia E. Ia, Ivkina N. S., Rapoport I. A. A-faktor, obespechivaiushchii biosintez streptomitsina mutantnym shtammom Actinomyces streptomycini. Dokl Akad Nauk SSSR. 1967 Nov-Dec;177(1):232–235. [PubMed] [Google Scholar]
- Kieser H. M., Kieser T., Hopwood D. A. A combined genetic and physical map of the Streptomyces coelicolor A3(2) chromosome. J Bacteriol. 1992 Sep;174(17):5496–5507. doi: 10.1128/jb.174.17.5496-5507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Amemura M., Nakata A. Nucleotide sequence of the phoR gene, a regulatory gene for the phosphate regulon of Escherichia coli. J Mol Biol. 1986 Dec 5;192(3):549–556. doi: 10.1016/0022-2836(86)90275-5. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. 1984 May 31-Jun 6Nature. 309(5967):462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray I. A., Gil J. A., Hopwood D. A., Shaw W. V. Nucleotide sequence of the chloramphenicol acetyltransferase gene of Streptomyces acrimycini. Gene. 1989 Dec 28;85(2):283–291. doi: 10.1016/0378-1119(89)90420-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stein D., Cohen S. N. A cloned regulatory gene of Streptomyces lividans can suppress the pigment deficiency phenotype of different developmental mutants. J Bacteriol. 1989 Apr;171(4):2258–2261. doi: 10.1128/jb.171.4.2258-2261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng H. C., Chen C. W. A cloned ompR-like gene of Streptomyces lividans 66 suppresses defective melC1, a putative copper-transfer gene. Mol Microbiol. 1991 May;5(5):1187–1196. doi: 10.1111/j.1365-2958.1991.tb01892.x. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Janssen G. R., Kieser T., Bibb M. J., Buttner M. J., Bibb M. J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986 Jun;203(3):468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]