Abstract
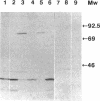
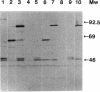
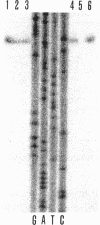
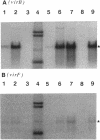
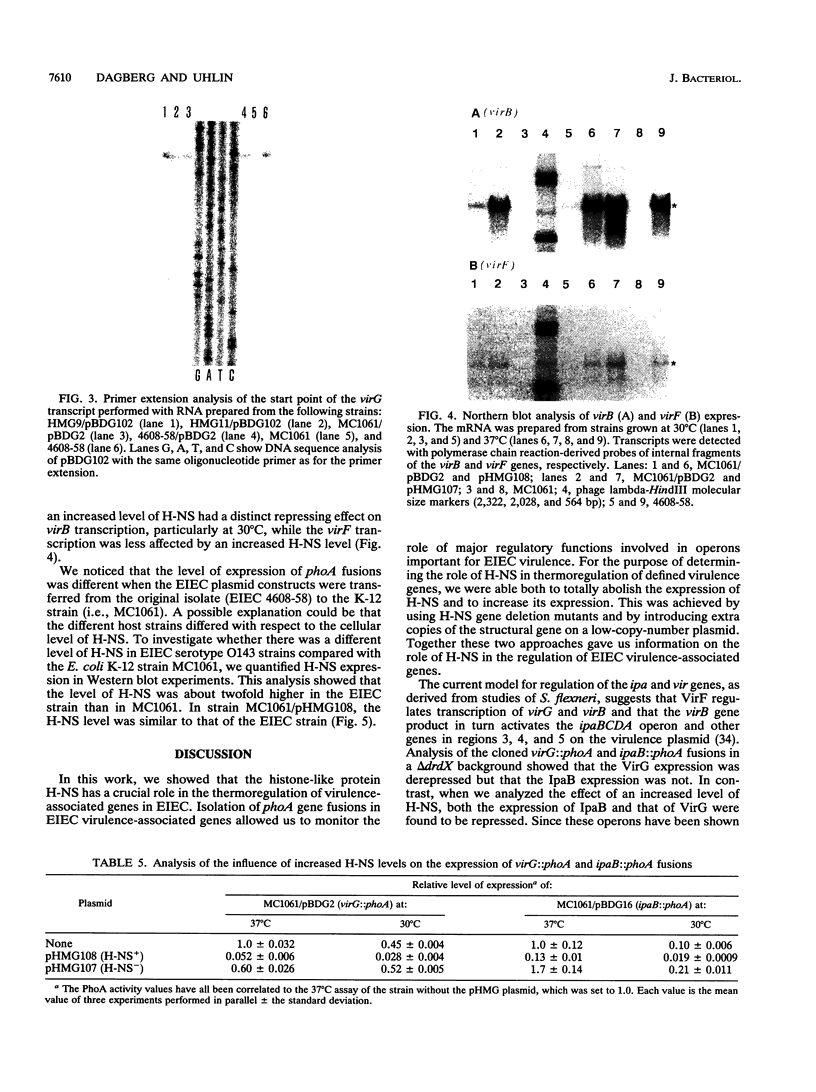
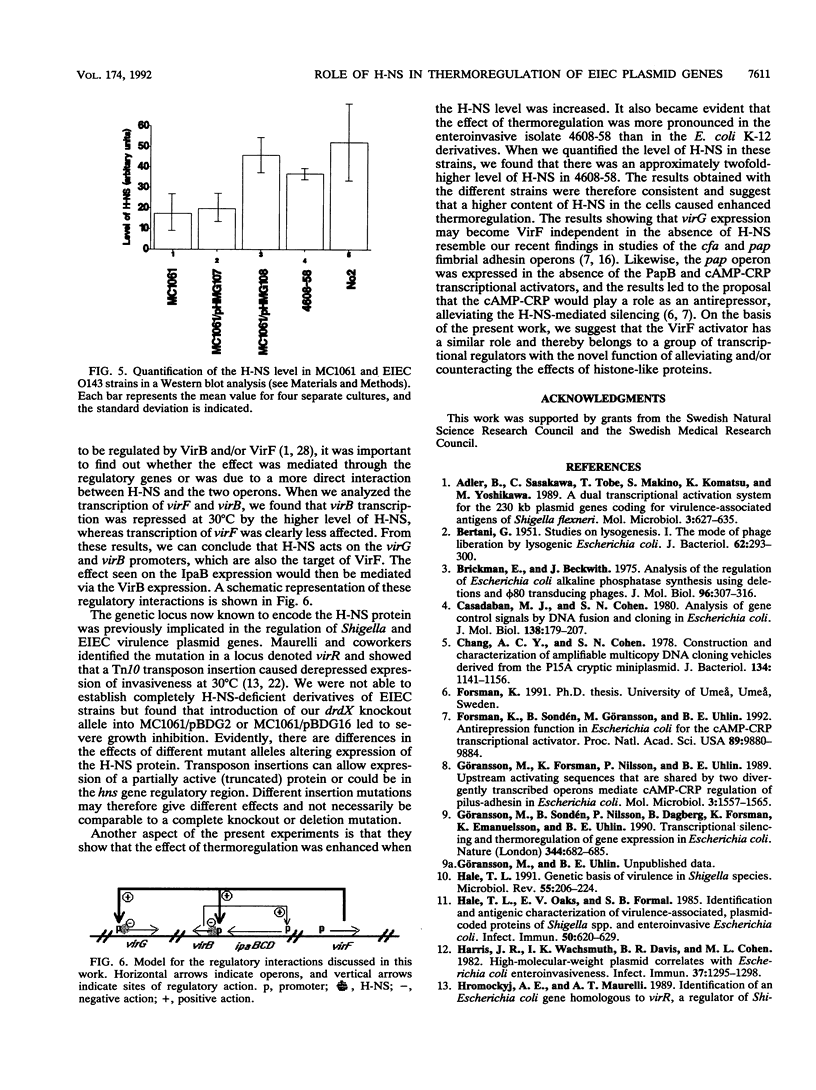
The transposon TnphoA was used for construction of gene fusions and for studies of gene regulation in an enteroinvasive strain of Escherichia coli. Several plasmid-encoded virulence genes (e.g., the ipaB and virG operons) of such enteroinvasive strains are subject to coordinated thermoregulation involving both operon-specific (the VirB and VirF activators) and global regulators. The nucleoid-associated E. coli protein H-NS was shown to be a negative regulator as judged by studies using H-NS gene deletion mutations and by increasing the level of H-NS protein in the cells. An increased gene dosage of H-NS led to enhanced repression of the ipa and virG operons, particularly at low (30 degrees C) growth temperature. The cyclic AMP receptor protein complex, which is another global transcriptional regulator in E. coli, was not required for the regulation of ipa and virG expression. The virG operon was expressed in an activator-independent manner in cells lacking H-NS protein. We suggest that the role of the VirF activator is to counteract the silencing effect of H-NS.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler B., Sasakawa C., Tobe T., Makino S., Komatsu K., Yoshikawa M. A dual transcriptional activation system for the 230 kb plasmid genes coding for virulence-associated antigens of Shigella flexneri. Mol Microbiol. 1989 May;3(5):627–635. doi: 10.1111/j.1365-2958.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman E., Beckwith J. Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and phi80 transducing phages. J Mol Biol. 1975 Aug 5;96(2):307–316. doi: 10.1016/0022-2836(75)90350-2. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman K., Sondén B., Göransson M., Uhlin B. E. Antirepression function in Escherichia coli for the cAMP-cAMP receptor protein transcriptional activator. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9880–9884. doi: 10.1073/pnas.89.20.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göransson M., Forsman P., Nilsson P., Uhlin B. E. Upstream activating sequences that are shared by two divergently transcribed operons mediate cAMP-CRP regulation of pilus-adhesin in Escherichia coli. Mol Microbiol. 1989 Nov;3(11):1557–1565. doi: 10.1111/j.1365-2958.1989.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Göransson M., Sondén B., Nilsson P., Dagberg B., Forsman K., Emanuelsson K., Uhlin B. E. Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature. 1990 Apr 12;344(6267):682–685. doi: 10.1038/344682a0. [DOI] [PubMed] [Google Scholar]
- Hale T. L. Genetic basis of virulence in Shigella species. Microbiol Rev. 1991 Jun;55(2):206–224. doi: 10.1128/mr.55.2.206-224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Oaks E. V., Formal S. B. Identification and antigenic characterization of virulence-associated, plasmid-coded proteins of Shigella spp. and enteroinvasive Escherichia coli. Infect Immun. 1985 Dec;50(3):620–629. doi: 10.1128/iai.50.3.620-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. R., Wachsmuth I. K., Davis B. R., Cohen M. L. High-molecular-weight plasmid correlates with Escherichia coli enteroinvasiveness. Infect Immun. 1982 Sep;37(3):1295–1298. doi: 10.1128/iai.37.3.1295-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Zabriskie J. B. Purification and partial characterization of the nephritis strain-associated protein from Streptococcus pyogenes, group A. J Exp Med. 1986 Mar 1;163(3):697–712. doi: 10.1084/jem.163.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordi B. J., Dagberg B., de Haan L. A., Hamers A. M., van der Zeijst B. A., Gaastra W., Uhlin B. E. The positive regulator CfaD overcomes the repression mediated by histone-like protein H-NS (H1) in the CFA/I fimbrial operon of Escherichia coli. EMBO J. 1992 Jul;11(7):2627–2632. doi: 10.1002/j.1460-2075.1992.tb05328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lett M. C., Sasakawa C., Okada N., Sakai T., Makino S., Yamada M., Komatsu K., Yoshikawa M. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J Bacteriol. 1989 Jan;171(1):353–359. doi: 10.1128/jb.171.1.353-359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski J. R., Ruiz A. A., Godson G. N. Promotion, termination, and anti-termination in the rpsU-dnaG-rpoD macromolecular synthesis operon of E. coli K-12. Mol Gen Genet. 1984;195(3):391–401. doi: 10.1007/BF00341439. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Sansonetti P. J. Identification of a chromosomal gene controlling temperature-regulated expression of Shigella virulence. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2820–2824. doi: 10.1073/pnas.85.8.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Inouye H., Oliver D., Beckwith J. Mutations that alter the signal sequence of alkaline phosphatase in Escherichia coli. J Bacteriol. 1983 Apr;154(1):366–374. doi: 10.1128/jb.154.1.366-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson P., Uhlin B. E. Differential decay of a polycistronic Escherichia coli transcript is initiated by RNaseE-dependent endonucleolytic processing. Mol Microbiol. 1991 Jul;5(7):1791–1799. doi: 10.1111/j.1365-2958.1991.tb01928.x. [DOI] [PubMed] [Google Scholar]
- Oaks E. V., Hale T. L., Formal S. B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986 Jul;53(1):57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pál T., Pácsa A. S., Emödy L., Vörös S., Sélley E. Modified enzyme-linked immunosorbent assay for detecting enteroinvasive Escherichia coli and virulent Shigella strains. J Clin Microbiol. 1985 Mar;21(3):415–418. doi: 10.1128/jcm.21.3.415-418.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Wolf-Watz H. Virulence plasmid-associated HeLa cell induced cytotoxicity of Yersinia pseudotuberculosis. Microb Pathog. 1986 Jun;1(3):229–240. doi: 10.1016/0882-4010(86)90047-1. [DOI] [PubMed] [Google Scholar]
- Sakai T., Sasakawa C., Makino S., Yoshikawa M. DNA sequence and product analysis of the virF locus responsible for congo red binding and cell invasion in Shigella flexneri 2a. Infect Immun. 1986 Nov;54(2):395–402. doi: 10.1128/iai.54.2.395-402.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Sasakawa C., Yoshikawa M. Expression of four virulence antigens of Shigella flexneri is positively regulated at the transcriptional level by the 30 kiloDalton virF protein. Mol Microbiol. 1988 Sep;2(5):589–597. doi: 10.1111/j.1365-2958.1988.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Murayama S. Y., Makino S., Yoshikawa M. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect Immun. 1986 Feb;51(2):470–475. doi: 10.1128/iai.51.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M. B. More than just "histone-like" proteins. Cell. 1990 Nov 2;63(3):451–453. doi: 10.1016/0092-8674(90)90438-k. [DOI] [PubMed] [Google Scholar]
- Silva R. M., Toledo M. R., Trabulsi L. R. Correlation of invasiveness with plasmid in enteroinvasive strains of Escherichia coli. J Infect Dis. 1982 Nov;146(5):706–706. doi: 10.1093/infdis/146.5.706. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Tobe T., Nagai S., Okada N., Adler B., Yoshikawa M., Sasakawa C. Temperature-regulated expression of invasion genes in Shigella flexneri is controlled through the transcriptional activation of the virB gene on the large plasmid. Mol Microbiol. 1991 Apr;5(4):887–893. doi: 10.1111/j.1365-2958.1991.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Kopecko D. J. Characterization of invasion plasmid antigen genes (ipaBCD) from Shigella flexneri. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9317–9321. doi: 10.1073/pnas.85.23.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]