Abstract
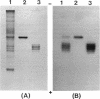
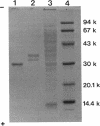
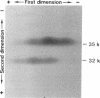
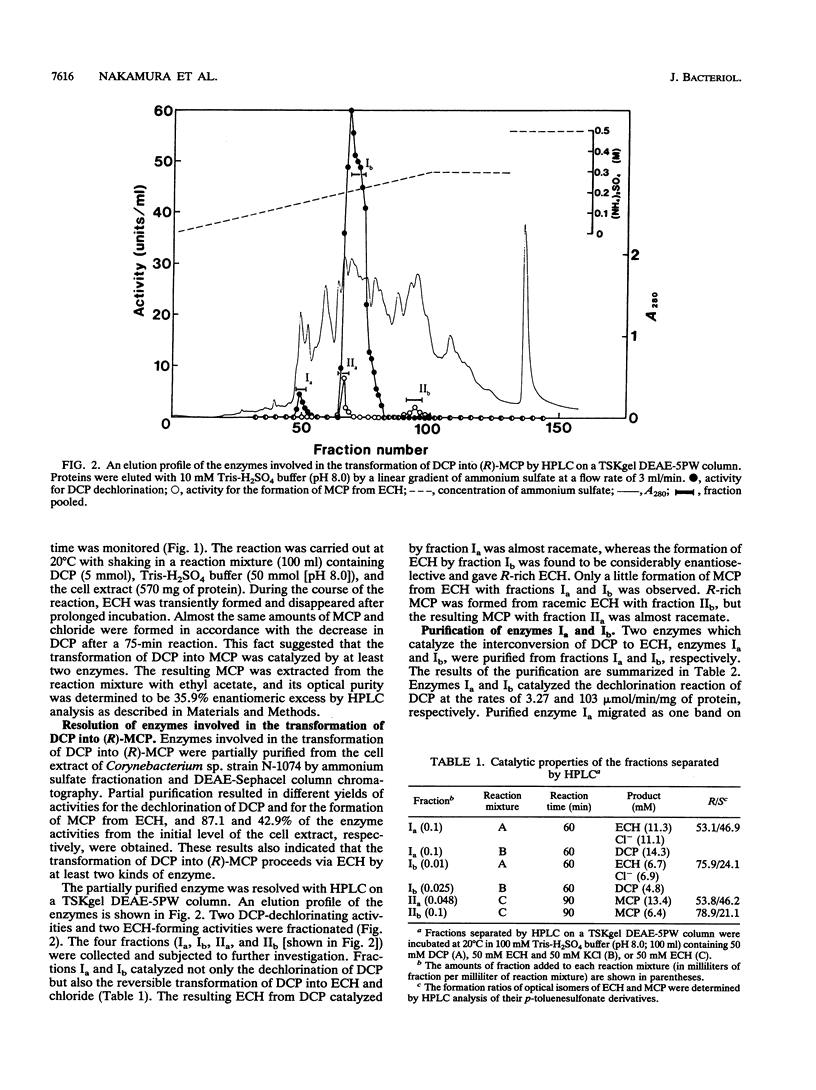
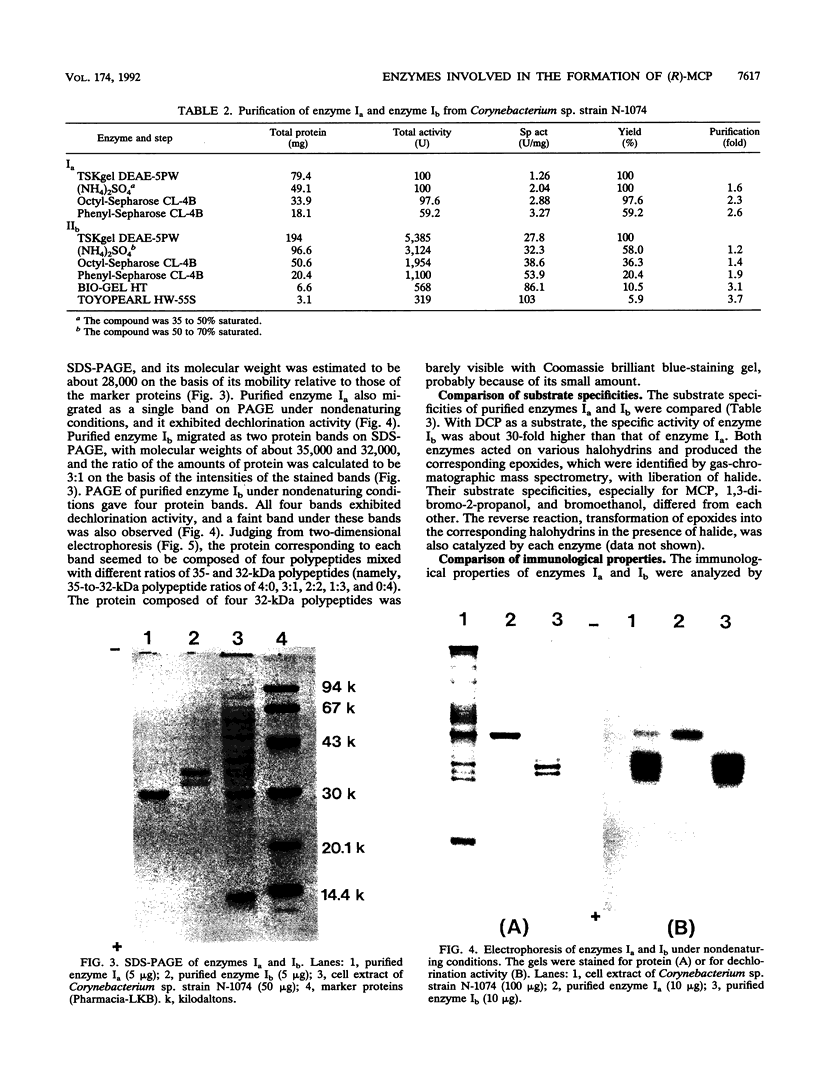
During the course of the transformation of 1,3-dichloro-2-propanol (DCP) into (R)-3-chloro-1,2-propanediol [(R)-MCP] with the cell extract of Corynebacterium sp. strain N-1074, epichlorohydrin (ECH) was transiently formed. The cell extract was fractionated into two DCP-dechlorinating activities (fractions Ia and Ib) and two ECH-hydrolyzing activities (fractions IIa and IIb) by TSKgel DEAE-5PW column chromatography. Fractions Ia and Ib catalyzed the interconversion of DCP to ECH, and fractions IIa and IIb catalyzed the transformation of ECH into MCP. Fractions Ia and IIa showed only low enantioselectivity for each reaction, whereas fractions Ib and IIb exhibited considerable enantioselectivity, yielding R-rich ECH and MCP, respectively. Enzymes Ia and Ib were isolated from fractions Ia and Ib, respectively. Enzyme Ia had a molecular mass of about 108 kDa and consisted of four subunits identical in molecular mass (about 28 kDa). Enzyme Ib was a protein of 115 kDa, composed of two different polypeptides (about 35 and 32 kDa). The specific activity of enzyme Ib for DCP was about 30-fold higher than that of enzyme Ia. Both enzymes catalyzed the transformation of several halohydrins into the corresponding epoxides with liberation of halides and its reverse reaction. Their substrate specificities and immunological properties differed from each other. Enzyme Ia seemed to be halohydrin hydrogen-halide-lyase which was already purified from Escherichia coli carrying a gene from Corynebacterium sp. strain N-1074.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castro C. E., Bartnicki E. W. Biodehalogenation. Epoxiation of halohydrins, epoxide opening, and transhalogenation by a Flavobacterium sp. Biochemistry. 1968 Sep;7(9):3213–3218. doi: 10.1021/bi00849a025. [DOI] [PubMed] [Google Scholar]
- Goldman P., Milne G. W., Keister D. B. Carbon-halogen bond cleavage. 3. Studies on bacterial halidohrolases. J Biol Chem. 1968 Jan 25;243(2):428–434. [PubMed] [Google Scholar]
- Janssen D. B., Gerritse J., Brackman J., Kalk C., Jager D., Witholt B. Purification and characterization of a bacterial dehalogenase with activity toward halogenated alkanes, alcohols and ethers. Eur J Biochem. 1988 Jan 15;171(1-2):67–72. doi: 10.1111/j.1432-1033.1988.tb13759.x. [DOI] [PubMed] [Google Scholar]
- Janssen D. B., Jager D., Witholt B. Degradation of n-haloalkanes and alpha, omega-dihaloalkanes by wild-type and mutants of Acinetobacter sp. strain GJ70. Appl Environ Microbiol. 1987 Mar;53(3):561–566. doi: 10.1128/aem.53.3.561-566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen D. B., Scheper A., Dijkhuizen L., Witholt B. Degradation of halogenated aliphatic compounds by Xanthobacter autotrophicus GJ10. Appl Environ Microbiol. 1985 Mar;49(3):673–677. doi: 10.1128/aem.49.3.673-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuning S., Janssen D. B., Witholt B. Purification and characterization of hydrolytic haloalkane dehalogenase from Xanthobacter autotrophicus GJ10. J Bacteriol. 1985 Aug;163(2):635–639. doi: 10.1128/jb.163.2.635-639.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Little M., Williams P. A. A bacterial halidohydrolase. Its purification, some properties and its modification by specific amino acid reagents. Eur J Biochem. 1971 Jul 15;21(1):99–109. doi: 10.1111/j.1432-1033.1971.tb01445.x. [DOI] [PubMed] [Google Scholar]
- Motosugi K., Esaki N., Soda K. Purification and properties of a new enzyme, DL-2-haloacid dehalogenase, from Pseudomonas sp. J Bacteriol. 1982 May;150(2):522–527. doi: 10.1128/jb.150.2.522-527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruisinger S., Klages U., Lingens F. Abbau der 4-chlorbenzoesäure durch eine Arthrobacter-Species. Arch Microbiol. 1976 Nov 2;110(23):253–256. doi: 10.1007/BF00690235. [DOI] [PubMed] [Google Scholar]
- Scholten J. D., Chang K. H., Babbitt P. C., Charest H., Sylvestre M., Dunaway-Mariano D. Novel enzymic hydrolytic dehalogenation of a chlorinated aromatic. Science. 1991 Jul 12;253(5016):182–185. doi: 10.1126/science.1853203. [DOI] [PubMed] [Google Scholar]
- Scholtz R., Leisinger T., Suter F., Cook A. M. Characterization of 1-chlorohexane halidohydrolase, a dehalogenase of wide substrate range from an Arthrobacter sp. J Bacteriol. 1987 Nov;169(11):5016–5021. doi: 10.1128/jb.169.11.5016-5021.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. M., Harrison K., Colby J. Purification and characterization of D-2-haloacid dehalogenase from Pseudomonas putida strain AJ1/23. J Gen Microbiol. 1990 May;136(5):881–886. doi: 10.1099/00221287-136-5-881. [DOI] [PubMed] [Google Scholar]
- Weightman A. J., Weightman A. L., Slater J. H. Stereospecificity of 2-monochloropropionate dehalogenation by the two dehalogenases of Pseudomonas putida PP3: evidence for two different dehalogenation mechanisms. J Gen Microbiol. 1982 Aug;128(8):1755–1762. doi: 10.1099/00221287-128-8-1755. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard A. J., Reuvekamp P. T., Janssen D. B. Purification and characterization of haloalcohol dehalogenase from Arthrobacter sp. strain AD2. J Bacteriol. 1991 Jan;173(1):124–129. doi: 10.1128/jb.173.1.124-129.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]