Abstract
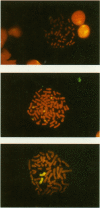
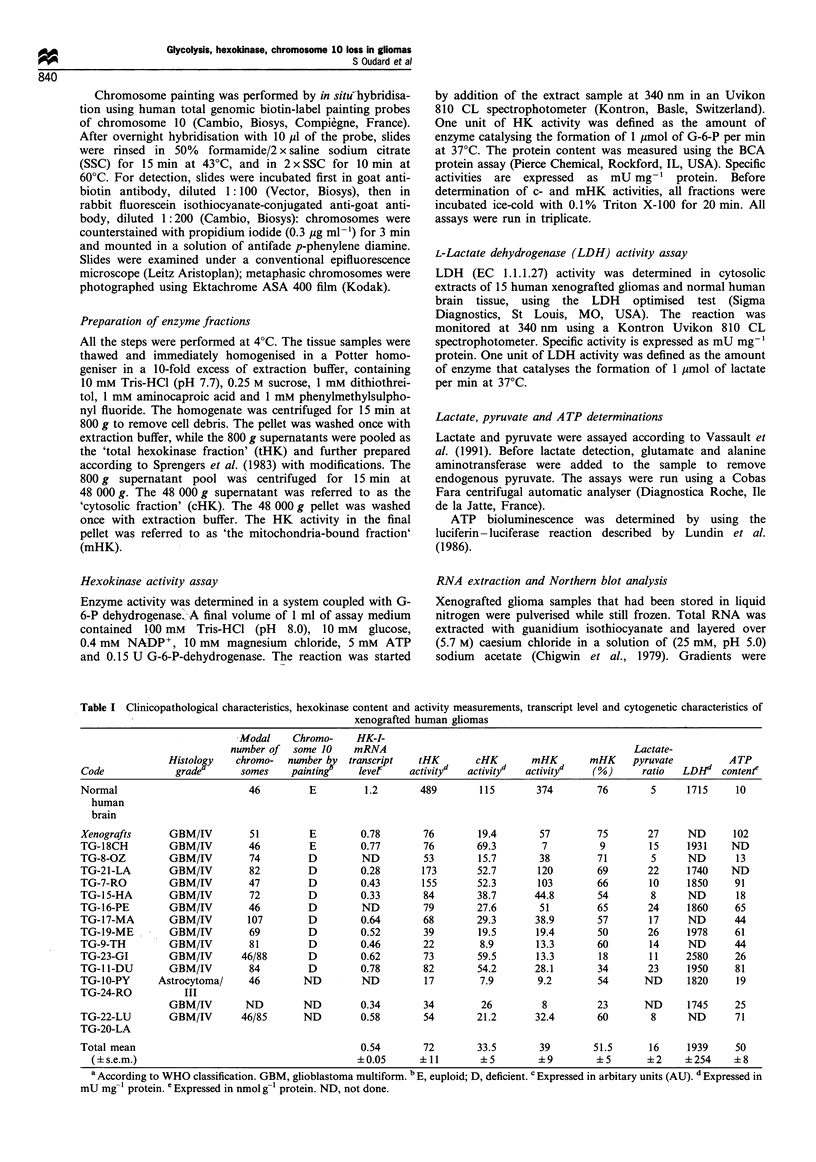
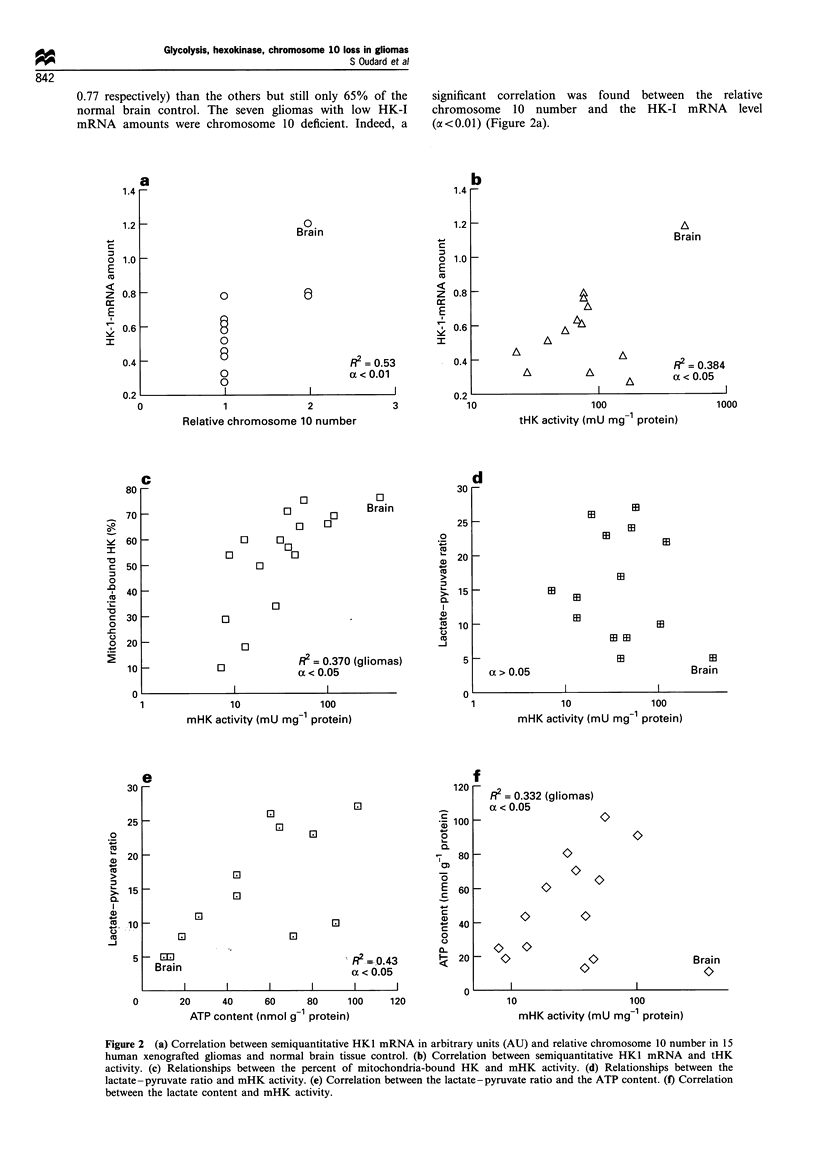
Loss of chromosome 10 was observed in 10 out of 12 xenografted glioblastomas studied. Chromosome 10 carries the gene coding the hexokinase type 1 isoenzyme (HK-I), which catalyses the first step of glycolysis, which is essential in brain tissue and glioblastomas. We investigated the relationships between the relative chromosome 10 number, the amount of HK-I mRNA, HK-I activity and its intracellular distribution, and glycolysis-related parameters such as the lactate-pyruvate ratio, lactate dehydrogenase (LDH) and ATP contents. Individual tumour HK-I mRNA amounts were 23-65% lower than that of normal human brain and reflected the relative decrease of chromosome 10 number (alpha < 0.01). Total HK activities of individual glioblastomas varied considerably but were constantly (a mean of seven times) lower than that of normal brain tissue. The mitochondria-bound HK-I fraction of individual tumours was generally over 50%, compared with that of normal brain tissue. As shown by lactate - pyruvate ratios, in all the gliomas, glycolysis was elevated to an average of 3-fold that measured in normal brain. An elevated ATP content was also constantly noted. Adaptation of glioblastoma metabolism to the chromosome 10 loss and to the HK-I transcription unit emphasises the critical role of glycolysis in their survival. We hypothesise that HK-I, the enzyme responsible for initiating glycolysis necessary for brain function, may approach its lowest limit in gliomas, thereby opening therapeutic access to pharmacological anti-metabolites affecting energy metabolism and tumour growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arora K. K., Pedersen P. L. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem. 1988 Nov 25;263(33):17422–17428. [PubMed] [Google Scholar]
- Bardot V., Luccioni C., Lefrançois D., Muleris M., Dutrillaux B. Activity of thymidylate synthetase, thymidine kinase and galactokinase in primary and xenografted human colorectal cancers in relation to their chromosomal patterns. Int J Cancer. 1991 Mar 12;47(5):670–674. doi: 10.1002/ijc.2910470507. [DOI] [PubMed] [Google Scholar]
- BeltrandelRio H., Wilson J. E. Hexokinase of rat brain mitochondria: relative importance of adenylate kinase and oxidative phosphorylation as sources of substrate ATP, and interaction with intramitochondrial compartments of ATP and ADP. Arch Biochem Biophys. 1991 Apr;286(1):183–194. doi: 10.1016/0003-9861(91)90026-f. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Bigner D. D. Cytogenetics of human brain tumors. Cancer Genet Cytogenet. 1990 Jul 15;47(2):141–154. doi: 10.1016/0165-4608(90)90024-5. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Burger P. C., Mahaley M. S., Jr, Bullard D. E., Muhlbaier L. H., Bigner D. D. Specific chromosomal abnormalities in malignant human gliomas. Cancer Res. 1988 Jan 15;48(2):405–411. [PubMed] [Google Scholar]
- Bigner S. H., Mark J. Chromosome and chromosomal progression of human gliomas in vivo, in vitro and in athymic nude mice. Prog Exp Tumor Res. 1984;27:67–82. doi: 10.1159/000408223. [DOI] [PubMed] [Google Scholar]
- Bondy M., Wiencke J., Wrensch M., Kyritsis A. P. Genetics of primary brain tumors: a review. J Neurooncol. 1994;18(1):69–81. doi: 10.1007/BF01324606. [DOI] [PubMed] [Google Scholar]
- Bravard A., Hoffschir F., Sabatier L., Ricoul M., Pinton A., Cassingena R., Estrade S., Luccioni C., Dutrillaux B. Early superoxide dismutase alterations during SV40-transformation of human fibroblasts. Int J Cancer. 1992 Nov 11;52(5):797–801. doi: 10.1002/ijc.2910520521. [DOI] [PubMed] [Google Scholar]
- Burger P. C. Malignant astrocytic neoplasms: classification, pathologic anatomy, and response to treatment. Semin Oncol. 1986 Mar;13(1):16–26. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Fanciulli M., Paggi M. G., Bruno T., Del Carlo C., Bonetto F., Gentile F. P., Floridi A. Glycolysis and growth rate in normal and in hexokinase-transfected NIH-3T3 cells. Oncol Res. 1994;6(9):405–409. [PubMed] [Google Scholar]
- Floridi A., Paggi M. G., Fanciulli M. Modulation of glycolysis in neuroepithelial tumors. J Neurosurg Sci. 1989 Jan-Mar;33(1):55–64. [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M., Fults D. W., Thomas G. A., Nakamura Y., Heilbrun M. P., White R., Story J. L., Naylor S. L., Kagan-Hallet K. S., Sheridan P. J. Loss of heterozygosity on chromosome 10 in human glioblastoma multiforme. Genomics. 1989 Feb;4(2):210–214. doi: 10.1016/0888-7543(89)90302-9. [DOI] [PubMed] [Google Scholar]
- Graham J. F., Cummins C. J., Smith B. H., Kornblith P. L. Regulation of hexokinase in cultured gliomas. Neurosurgery. 1985 Oct;17(4):537–542. doi: 10.1227/00006123-198510000-00001. [DOI] [PubMed] [Google Scholar]
- Hoffschir F., Vuillaume M., Sabatier L., Ricoul M., Daya-Grosjean L., Estrade S., Cassingena R., Calvayrac R., Sarasin A., Dutrillaux B. Decrease in catalase activity and loss of the 11p chromosome arm in the course of SV40 transformation of human fibroblasts. Carcinogenesis. 1993 Aug;14(8):1569–1572. doi: 10.1093/carcin/14.8.1569. [DOI] [PubMed] [Google Scholar]
- James C. D., Carlbom E., Dumanski J. P., Hansen M., Nordenskjold M., Collins V. P., Cavenee W. K. Clonal genomic alterations in glioma malignancy stages. Cancer Res. 1988 Oct 1;48(19):5546–5551. [PubMed] [Google Scholar]
- Lowry O. H., Berger S. J., Carter J. G., Chi M. M., Manchester J. K., Knor J., Pusateri M. E. Diversity of metabolic patterns in human brain tumors: enzymes of energy metabolism and related metabolites and cofactors. J Neurochem. 1983 Oct;41(4):994–1010. doi: 10.1111/j.1471-4159.1983.tb09043.x. [DOI] [PubMed] [Google Scholar]
- Lundin A., Hasenson M., Persson J., Pousette A. Estimation of biomass in growing cell lines by adenosine triphosphate assay. Methods Enzymol. 1986;133:27–42. doi: 10.1016/0076-6879(86)33053-2. [DOI] [PubMed] [Google Scholar]
- MACBETH R. A., BEKESI J. G. Oxygen consumption and anaerobic glycolysis of human malignant and normal tissue. Cancer Res. 1962 Feb;22:244–248. [PubMed] [Google Scholar]
- Magdelénat H., Gerbault-Seureau M., Dutrillaux B. Relationship between loss of estrogen and progesterone receptor expression and of 6q and 11q chromosome arms in breast cancer. Int J Cancer. 1994 Apr 1;57(1):63–66. doi: 10.1002/ijc.2910570112. [DOI] [PubMed] [Google Scholar]
- Miccoli L., Oudard S., Sureau F., Poirson F., Dutrillaux B., Poupon M. F. Intracellular pH governs the subcellular distribution of hexokinase in a glioma cell line. Biochem J. 1996 Feb 1;313(Pt 3):957–962. doi: 10.1042/bj3130957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., Seino S., Bell G. I. Human hexokinase: sequences of amino- and carboxyl-terminal halves are homologous. Biochem Biophys Res Commun. 1988 Dec 30;157(3):937–943. doi: 10.1016/s0006-291x(88)80964-1. [DOI] [PubMed] [Google Scholar]
- Oudard S., Poirson F., Miccoli L., Bourgeois Y., Vassault A., Poisson M., Magdelénat H., Dutrillaux B., Poupon M. F. Mitochondria-bound hexokinase as target for therapy of malignant gliomas. Int J Cancer. 1995 Jul 17;62(2):216–222. doi: 10.1002/ijc.2910620218. [DOI] [PubMed] [Google Scholar]
- Parry D. M., Pedersen P. L. Glucose catabolism in brain. Intracellular localization of hexokinase. J Biol Chem. 1990 Jan 15;265(2):1059–1066. [PubMed] [Google Scholar]
- Pedersen P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sprengers E. D., Koenderman A. H., Staal G. E. Mitochondrial and cytosolic hexokinase from rat brain: one and the same enzyme? Biochim Biophys Acta. 1983 Jan 4;755(1):112–118. doi: 10.1016/0304-4165(83)90280-5. [DOI] [PubMed] [Google Scholar]
- Steck P. A., Ligon A. H., Cheong P., Yung W. K., Pershouse M. A. Two tumor suppressive loci on chromosome 10 involved in human glioblastomas. Genes Chromosomes Cancer. 1995 Apr;12(4):255–261. doi: 10.1002/gcc.2870120404. [DOI] [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Nagai M., Wakai S., Arai T., Kawashima K. Loss of constitutional heterozygosity in chromosome 10 in human glioblastoma. Acta Neuropathol. 1990;80(3):251–254. doi: 10.1007/BF00294641. [DOI] [PubMed] [Google Scholar]
- Wilson J. E. Hexokinases. Rev Physiol Biochem Pharmacol. 1995;126:65–198. doi: 10.1007/BFb0049776. [DOI] [PubMed] [Google Scholar]