Abstract
Primary chemotherapy in operable breast invasive carcinoma enables tumour reduction and conservative surgery. In order to search for one or more biological factors capable of predicting tumour behaviour under primary chemotherapy, and subsequent patient survival, an immunohistochemical study was performed with specific antibodies to p53, c-erbB-2 (Her-2/neu), Mib1 (antiKi-67), pS2, GST pi, oestrogen receptors (ERs) and progesterone receptors (PRs). Core biopsies, obtained before primary chemotherapy, were available from a series of 128 breast invasive carcinomas treated between January 1985 and April 1989, with a median follow-up of 93.3 months. Univariate statistical analysis showed that negative ER detection by immunohistochemistry (IHC) was highly correlated with chemosensitivity (P = 0.001). A high percentage of Mib1-positive tumour cells (> 40%), as well as initial tumour size less than 4 cm, were also correlated with tumour responsiveness to chemotherapy (P = 0.009 and P = 0.03). By multivariate analysis IHC-ER, Mib1 and initial tumour size were independent predictors, the last parameter being the most important. Concerning subsequent patient survival, c-erbB-2 overexpression, as detected by IHC, was significant with respect to overall survival (OS) (P = 0.0006), disease-free interval (DFI) (P = 0.03) and metastasis-free interval (MFI) (P = 0.008) by univariate analysis. Furthermore, c-erbB-2 was the major independent prognostic factor for OS and MFI by multivariate analysis.
Full text
PDF


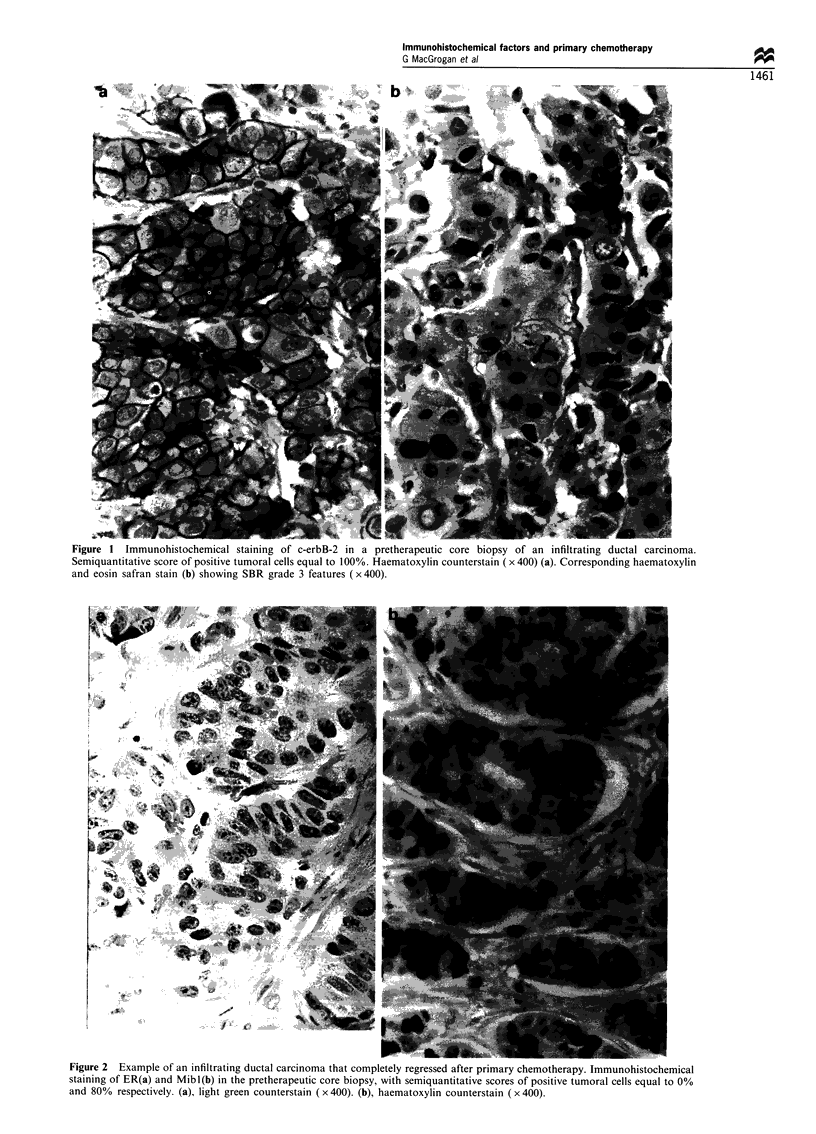




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. C., Clark G. M., Tandon A. K., Molina R., Tormey D. C., Osborne C. K., Gilchrist K. W., Mansour E. G., Abeloff M., Eudey L. HER-2/neu in node-negative breast cancer: prognostic significance of overexpression influenced by the presence of in situ carcinoma. J Clin Oncol. 1992 Apr;10(4):599–605. doi: 10.1200/JCO.1992.10.4.599. [DOI] [PubMed] [Google Scholar]
- Bonadonna G., Veronesi U., Brambilla C., Ferrari L., Luini A., Greco M., Bartoli C., Coopmans de Yoldi G., Zucali R., Rilke F. Primary chemotherapy to avoid mastectomy in tumors with diameters of three centimeters or more. J Natl Cancer Inst. 1990 Oct 3;82(19):1539–1545. doi: 10.1093/jnci/82.19.1539. [DOI] [PubMed] [Google Scholar]
- Bélembaogo E., Feillel V., Chollet P., Curé H., Verrelle P., Kwiatkowski F., Achard J. L., Le Bouëdec G., Chassagne J., Bignon Y. J. Neoadjuvant chemotherapy in 126 operable breast cancers. Eur J Cancer. 1992;28A(4-5):896–900. doi: 10.1016/0959-8049(92)90145-r. [DOI] [PubMed] [Google Scholar]
- Calais G., Berger C., Descamps P., Chapet S., Reynaud-Bougnoux A., Body G., Bougnoux P., Lansac J., Le Floch O. Conservative treatment feasibility with induction chemotherapy, surgery, and radiotherapy for patients with breast carcinoma larger than 3 cm. Cancer. 1994 Aug 15;74(4):1283–1288. doi: 10.1002/1097-0142(19940815)74:4<1283::aid-cncr2820740417>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Daidone M. G., Silvestrini R., Valentinis B., Ferrari L., Bartoli C. Changes in cell kinetics induced by primary chemotherapy in breast cancer. Int J Cancer. 1991 Feb 1;47(3):380–383. doi: 10.1002/ijc.2910470312. [DOI] [PubMed] [Google Scholar]
- Drewinko B., Patchen M., Yang L. Y., Barlogie B. Differential killing efficacy of twenty antitumor drugs on proliferating and nonproliferating human tumor cells. Cancer Res. 1981 Jun;41(6):2328–2333. [PubMed] [Google Scholar]
- Feldman L. D., Hortobagyi G. N., Buzdar A. U., Ames F. C., Blumenschein G. R. Pathological assessment of response to induction chemotherapy in breast cancer. Cancer Res. 1986 May;46(5):2578–2581. [PubMed] [Google Scholar]
- Gardin G., Alama A., Rosso R., Campora E., Repetto L., Pronzato P., Merlini L., Naso C., Camoriano A., Meazza R. Relationship of variations in tumor cell kinetics induced by primary chemotherapy to tumor regression and prognosis in locally advanced breast cancer. Breast Cancer Res Treat. 1994;32(3):311–318. doi: 10.1007/BF00666008. [DOI] [PubMed] [Google Scholar]
- Gasparini G., Gullick W. J., Bevilacqua P., Sainsbury J. R., Meli S., Boracchi P., Testolin A., La Malfa G., Pozza F. Human breast cancer: prognostic significance of the c-erbB-2 oncoprotein compared with epidermal growth factor receptor, DNA ploidy, and conventional pathologic features. J Clin Oncol. 1992 May;10(5):686–695. doi: 10.1200/JCO.1992.10.5.686. [DOI] [PubMed] [Google Scholar]
- Gazet J. C., Ford H. T., Coombes R. C. Randomised trial of chemotherapy versus endocrine therapy in patients presenting with locally advanced breast cancer (a pilot study). Br J Cancer. 1991 Feb;63(2):279–282. doi: 10.1038/bjc.1991.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquillat C., Weil M., Baillet F., Borel C., Auclerc G., de Maublanc M. A., Housset M., Forget G., Thill L., Soubrane C. Results of neoadjuvant chemotherapy and radiation therapy in the breast-conserving treatment of 250 patients with all stages of infiltrative breast cancer. Cancer. 1990 Jul 1;66(1):119–129. doi: 10.1002/1097-0142(19900701)66:1<119::aid-cncr2820660122>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Klijn J. G., Berns E. M., Foekens J. A. Prognostic factors and response to therapy in breast cancer. Cancer Surv. 1993;18:165–198. [PubMed] [Google Scholar]
- Livingston R. B. Breast cancer and response to chemotherapy: a possible relationship of hormone receptors and doxorubicin. Cancer Treat Rev. 1982 Sep;9(3):229–236. doi: 10.1016/s0305-7372(82)80008-x. [DOI] [PubMed] [Google Scholar]
- MacGrogan G., Bonichon F., de Mascarel I., Trojani M., Durand M., Avril A., Coindre J. M. Prognostic value of p53 in breast invasive ductal carcinoma: an immunohistochemical study on 942 cases. Breast Cancer Res Treat. 1995;36(1):71–81. doi: 10.1007/BF00690187. [DOI] [PubMed] [Google Scholar]
- Mauriac L., Durand M., Avril A., Dilhuydy J. M. Effects of primary chemotherapy in conservative treatment of breast cancer patients with operable tumors larger than 3 cm. Results of a randomized trial in a single centre. Ann Oncol. 1991 May;2(5):347–354. doi: 10.1093/oxfordjournals.annonc.a057953. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Rao B. R., Stevens S. C., White W. L. Low incidence of estrogen receptor in breast carcinomas with rapid rates of cellular replication. Cancer. 1977 Nov;40(5):2290–2298. doi: 10.1002/1097-0142(197711)40:5<2290::aid-cncr2820400541>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Mortimer J., Flournoy N., Livingston R. B., Stephens R. L. Aggressive adriamycin-containing regimen (PM-FAC) in estrogen receptor-negative disseminated breast cancer. Results of a Southwest Oncology Group trial. Cancer. 1985 Nov 15;56(10):2376–2380. doi: 10.1002/1097-0142(19851115)56:10<2376::aid-cncr2820561005>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Muss H. B., Thor A. D., Berry D. A., Kute T., Liu E. T., Koerner F., Cirrincione C. T., Budman D. R., Wood W. C., Barcos M. c-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med. 1994 May 5;330(18):1260–1266. doi: 10.1056/NEJM199405053301802. [DOI] [PubMed] [Google Scholar]
- O'Reilly S. M., Camplejohn R. S., Rubens R. D., Richards M. A. DNA flow cytometry and response to preoperative chemotherapy for primary breast cancer. Eur J Cancer. 1992;28(2-3):681–683. doi: 10.1016/s0959-8049(05)80124-8. [DOI] [PubMed] [Google Scholar]
- Quénel N., Wafflart J., Bonichon F., de Mascarel I., Trojani M., Durand M., Avril A., Coindre J. M. The prognostic value of c-erbB2 in primary breast carcinomas: a study on 942 cases. Breast Cancer Res Treat. 1995 Sep;35(3):283–291. doi: 10.1007/BF00665980. [DOI] [PubMed] [Google Scholar]
- Raemaekers J. M., Beex L. V., Pieters G. F., Smals A. G., Benraad T. J., Kloppenborg P. W. Progesterone receptor activity and relapse-free survival in patients with primary breast cancer: the role of adjuvant chemotherapy. Breast Cancer Res Treat. 1987;9(3):191–199. doi: 10.1007/BF01806379. [DOI] [PubMed] [Google Scholar]
- Rasbridge S. A., Gillett C. E., Seymour A. M., Patel K., Richards M. A., Rubens R. D., Millis R. R. The effects of chemotherapy on morphology, cellular proliferation, apoptosis and oncoprotein expression in primary breast carcinoma. Br J Cancer. 1994 Aug;70(2):335–341. doi: 10.1038/bjc.1994.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remvikos Y., Mosseri V., Zajdela A., Fourquet A., Durand J. C., Pouillart P., Magdelénat H. Prognostic value of the S-phase fraction of breast cancers treated by primary radiotherapy or neoadjuvant chemotherapy. Ann N Y Acad Sci. 1993 Nov 30;698:193–203. doi: 10.1111/j.1749-6632.1993.tb17209.x. [DOI] [PubMed] [Google Scholar]
- Scholl S. M., Asselain B., Palangie T., Dorval T., Jouve M., Garcia Giralt E., Vilcoq J., Durand J. C., Pouillart P. Neoadjuvant chemotherapy in operable breast cancer. Eur J Cancer. 1991;27(12):1668–1671. doi: 10.1016/0277-5379(91)90442-g. [DOI] [PubMed] [Google Scholar]
- Silverstrini R., Daidone M. G., Di Fronzo G. Relationship between proliferative activity and estrogen receptors in breast cancer. Cancer. 1979 Aug;44(2):665–670. doi: 10.1002/1097-0142(197908)44:2<665::aid-cncr2820440237>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Simon R., Altman D. G. Statistical aspects of prognostic factor studies in oncology. Br J Cancer. 1994 Jun;69(6):979–985. doi: 10.1038/bjc.1994.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. E., Walsh G., Jones A., Prendiville J., Johnston S., Gusterson B., Ramage F., Robertshaw H., Sacks N., Ebbs S. High complete remission rates with primary neoadjuvant infusional chemotherapy for large early breast cancer. J Clin Oncol. 1995 Feb;13(2):424–429. doi: 10.1200/JCO.1995.13.2.424. [DOI] [PubMed] [Google Scholar]
- Soubeyran I., Wafflart J., Bonichon F., de Mascarel I., Trojani M., Durand M., Avril A., Coindre J. M. Immunohistochemical determination of pS2 in invasive breast carcinomas: a study on 942 cases. Breast Cancer Res Treat. 1995 May;34(2):119–128. doi: 10.1007/BF00665784. [DOI] [PubMed] [Google Scholar]
- Spyratos F., Briffod M., Tubiana-Hulin M., Andrieu C., Mayras C., Pallud C., Lasry S., Rouëssé J. Sequential cytopunctures during preoperative chemotherapy for primary breast carcinoma. II. DNA flow cytometry changes during chemotherapy, tumor regression, and short-term follow-up. Cancer. 1992 Jan 15;69(2):470–475. doi: 10.1002/1097-0142(19920115)69:2<470::aid-cncr2820690233>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Stål O., Skoog L., Rutqvist L. E., Carstensen J. M., Wingren S., Sullivan S., Andersson A. C., Dufmats M., Nordenskjöld B. S-phase fraction and survival benefit from adjuvant chemotherapy or radiotherapy of breast cancer. Br J Cancer. 1994 Dec;70(6):1258–1262. doi: 10.1038/bjc.1994.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. Cell kinetics and chemotherapy: a critical review. Cancer Treat Rep. 1978 Aug;62(8):1117–1133. [PubMed] [Google Scholar]
- Weichselbaum R. R., Hellman S., Piro A. J., Nove J. J., Little J. B. Proliferation kinetics of a human breast cancer line in vitro following treatment with 17beta-estradiol and 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1978 Aug;38(8):2339–2342. [PubMed] [Google Scholar]
- Whelan R. D., Hosking L. K., Townsend A. J., Cowan K. H., Hill B. T. Differential increases in glutathione S-transferase activities in a range of multidrug-resistant human tumor cell lines. Cancer Commun. 1989;1(6):359–365. doi: 10.3727/095535489820875057. [DOI] [PubMed] [Google Scholar]
- Whelan R. D., Waring C. J., Wolf C. R., Hayes J. D., Hosking L. K., Hill B. T. Over-expression of P-glycoprotein and glutathione S-transferase pi in MCF-7 cells selected for vincristine resistance in vitro. Int J Cancer. 1992 Sep 9;52(2):241–246. doi: 10.1002/ijc.2910520215. [DOI] [PubMed] [Google Scholar]




