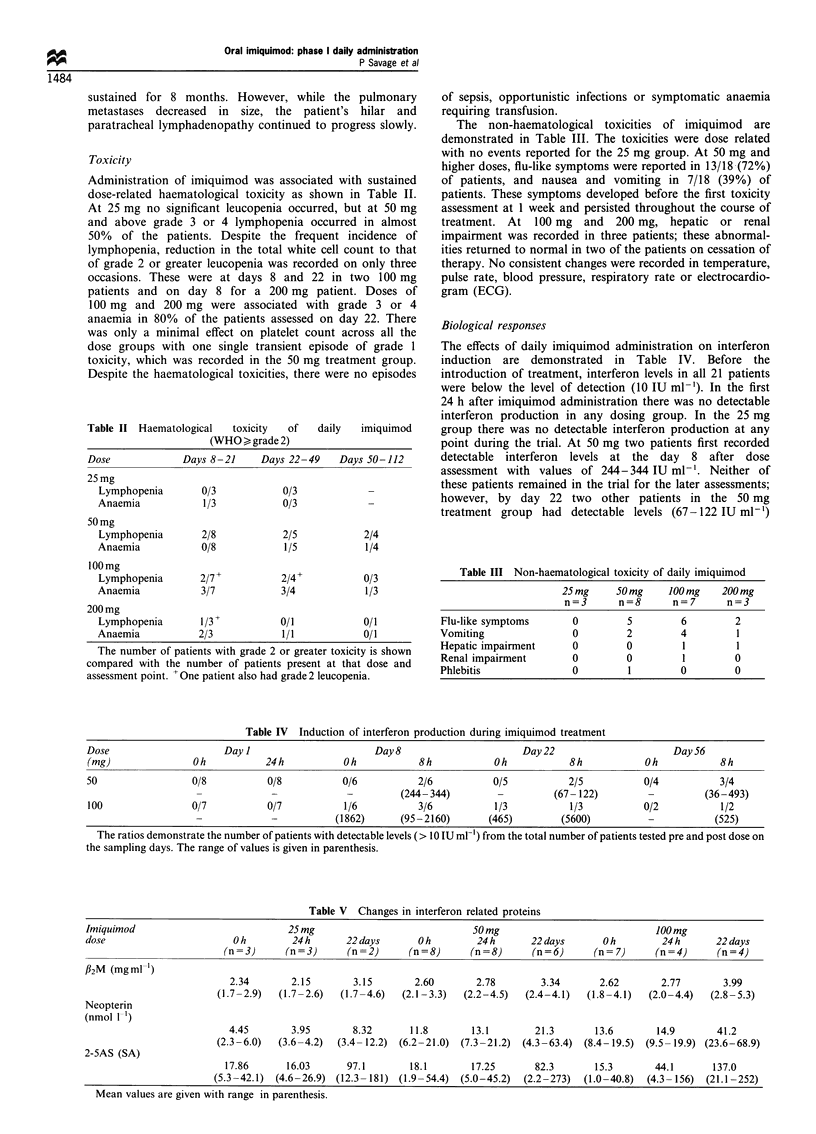
Abstract
Imiquimod is an orally active interferon inducer with anti-tumour activity in experimental animals. In this study the tolerability, toxicity and biological effects of daily oral imiquimod administration were investigated in 21 patients with refractory cancer. Patients were treated with doses of 25 mg, 50 mg, 100 mg or 200 mg on a projected 112 day course. Only three patients completed the course, all at the 50 mg dose. Treatment toxicities were dose related and mainly comprised flu-like symptoms, nausea and lymphopenia. Of the 21 patients, five received dose reductions and in five treatment was discontinued because of treatment-related toxicity. The biological activity of imiquimod was confirmed by significant and sustained rises in peripheral blood mononuclear cell (PBMC) 2-5A synthetase (2-5AS) levels at all doses. At 100 mg and 200 mg these occurred within the first 24 h of administration. Levels of neopterin and beta 2-microglobulin (beta 2M) were also significantly elevated when assessed after three weeks' treatment. Interferon production was not demonstrated within the first 24 h of the initial dose but, following repeated doses, ten of the patients developed detectable serum interferon concentrations with a maximum value of 5600 IU ml-1 recorded. Administration of imiquimod did not have any significant effect on serum levels of tumour necrosis factor (TNF) or interleukin 1 (IL-1), nor did it lead to development of detectable levels of antibodies to interferon. One mixed clinical response was observed after 4 weeks' treatment at 100 mg in a patient with renal cell cancer. Daily administration of imiquimod causes activation of the interferon production system but at higher doses results in unacceptable toxicity. Further investigation of imiquimod as an interferon-inducing agent in cancer patients is suggested at either the lower dose levels or employing alternative dosing schedules.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dianzani F. Interferon treatments: how to use an endogenous system as a therapeutic agent. J Interferon Res. 1992 May;Spec No:109–118. doi: 10.1089/jir.1992.1992.109. [DOI] [PubMed] [Google Scholar]
- Horoszewicz J. S., Murphy G. P. An assessment of the current use of human interferons in therapy of urological cancers. J Urol. 1989 Nov;142(5):1173–1180. doi: 10.1016/s0022-5347(17)39022-5. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Youn J. K., Buffet-Janvresse C., Riviere Y., Michelson M., Lacour J., Lacour F. Enhancement of natural killer cell activity and 2-5A synthetase in operable breast cancer patients treated with polyadenylic; polyuridylic acid. Cancer. 1985 Jan 15;55(2):357–362. doi: 10.1002/1097-0142(19850115)55:2<357::aid-cncr2820550210>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Mayer G. D., Krueger R. F. Tilorone hydrochloride: mode of action. Science. 1970 Sep 18;169(3951):1214–1215. doi: 10.1126/science.169.3951.1214. [DOI] [PubMed] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981 Jan 1;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Nichol F. R., Weed S. D., Underwood G. E. Stimulation of murine interferon by a substituted pyrimidine. Antimicrob Agents Chemother. 1976 Mar;9(3):433–439. doi: 10.1128/aac.9.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada J. R., Reuben J., Manning J. T., Hersh E. M., Gutterman J. U. Alpha interferon for induction of remission in hairy-cell leukemia. N Engl J Med. 1984 Jan 5;310(1):15–18. doi: 10.1056/NEJM198401053100104. [DOI] [PubMed] [Google Scholar]
- Reiter M. J., Testerman T. L., Miller R. L., Weeks C. E., Tomai M. A. Cytokine induction in mice by the immunomodulator imiquimod. J Leukoc Biol. 1994 Feb;55(2):234–240. doi: 10.1002/jlb.55.2.234. [DOI] [PubMed] [Google Scholar]
- Rios A., Stringfellow D. A., Fitzpatrick F. A., Reele S. B., Gutknecht G. D., Hersh E. M. Phase I study of 2-amino-5-bromo-6-phenyl-4(3H)-pyrimidinone (ABPP), an oral interferon inducer, in cancer patients. J Biol Response Mod. 1986 Aug;5(4):330–338. [PubMed] [Google Scholar]
- Sarosdy M. F., Lamm D. L., Williams R. D., Moon T. D., Flanigan R. C., Crawford E. D., Wilks N. E., Earhart R. H., Merritt J. A. Phase 1 trial of oral bropirimine in superficial bladder cancer. J Urol. 1992 Jan;147(1):31–33. doi: 10.1016/s0022-5347(17)37126-4. [DOI] [PubMed] [Google Scholar]
- Sidky Y. A., Borden E. C., Weeks C. E., Reiter M. J., Hatcher J. F., Bryan G. T. Inhibition of murine tumor growth by an interferon-inducing imidazoquinolinamine. Cancer Res. 1992 Jul 1;52(13):3528–3533. [PubMed] [Google Scholar]
- Steis R. G., Smith J. W., 2nd, Urba W. J., Clark J. W., Itri L. M., Evans L. M., Schoenberger C., Longo D. L. Resistance to recombinant interferon alfa-2a in hairy-cell leukemia associated with neutralizing anti-interferon antibodies. N Engl J Med. 1988 Jun 2;318(22):1409–1413. doi: 10.1056/NEJM198806023182201. [DOI] [PubMed] [Google Scholar]
- Stringfellow D. A., Weed S. D., Underwood G. E. Antiviral and interferon-inducing properties of 1,5-diamino anthraquinones. Antimicrob Agents Chemother. 1979 Jan;15(1):111–118. doi: 10.1128/aac.15.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks C. E., Gibson S. J. Induction of interferon and other cytokines by imiquimod and its hydroxylated metabolite R-842 in human blood cells in vitro. J Interferon Res. 1994 Apr;14(2):81–85. doi: 10.1089/jir.1994.14.81. [DOI] [PubMed] [Google Scholar]
- Witt P. L., Ritch P. S., Reding D., McAuliffe T. L., Westrick L., Grossberg S. E., Borden E. C. Phase I trial of an oral immunomodulator and interferon inducer in cancer patients. Cancer Res. 1993 Nov 1;53(21):5176–5180. [PubMed] [Google Scholar]
- von Wussow P., Block B., Hartmann F., Deicher H. Intralesional interferon-alpha therapy in advanced malignant melanoma. Cancer. 1988 Mar 15;61(6):1071–1074. doi: 10.1002/1097-0142(19880315)61:6<1071::aid-cncr2820610603>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]



