Abstract
The monoclonal anti-CEA antibody, A5B7, has previously been administered to patients for radioimmunotherapy (RIT). Long circulation time and the formation of an immune response have limited therapeutic success in the clinic. Antibody fragments can be used to reduce the in vivo circulation time, but the best combination of fragment and radioisotope to use for therapy is far from clear. In this study we have compared the biodistribution of A5B7 IgG and F(ab')2 with chemically cross-linked divalent (DFM) and trivalent (TFM) A5B7 Fab' fragments in nude mice bearing human colorectal tumour xenografts. The cross-linkers were designed to allow site-specific labelling using yttrium 90 (90Y), a high-energy beta-emitter. We have also compared the above antibody forms conjugated to both 131I and 90Y. Both DFM and TFM were fully immunoreactive and remained intact after radiolabelling and incubation in serum at 37 degrees C for 24 h. Biodistribution results showed similar tumour uptake levels and an identical blood clearance pattern for F(ab')2 and DFM with high tumour-blood ratios generated in each case. However, unacceptably high kidney accumulation for both F(ab')2 and DFM and elevated splenic uptake of DFM labelled with 90Y was observed. Kinetic analysis of antigen binding revealed that DFM had the fastest association rate (kass = 1.6 x 10(5) Ms-1) of the antibody forms, perhaps owing to increased flexibility of the cross-linker. This advantage implies that DFM may be more suitable than F(ab')2 radiolabelled with 131I for RIT. TFM cleared from the blood significantly faster than A5B7 IgG when labelled with both 131I and 90Y, producing an improved therapeutic tumour-blood ratio. Kidney accumulation was not observed for [90Y]TFM, but a slightly higher splenic uptake was observed that may indicate reticuloendothelial system (RES) uptake. Overall, tumour uptake was higher for 90Y-labelled antibodies than for 131I-labelled antibodies. Because of the faster clearance, it should be possible to administer a higher total dose of 90Y-labelled TFM than IgG, which is attractive for RIT. Both A5B7 DFM and TFM, therefore, show favourable properties compared with their parent antibody forms.
Full text
PDF

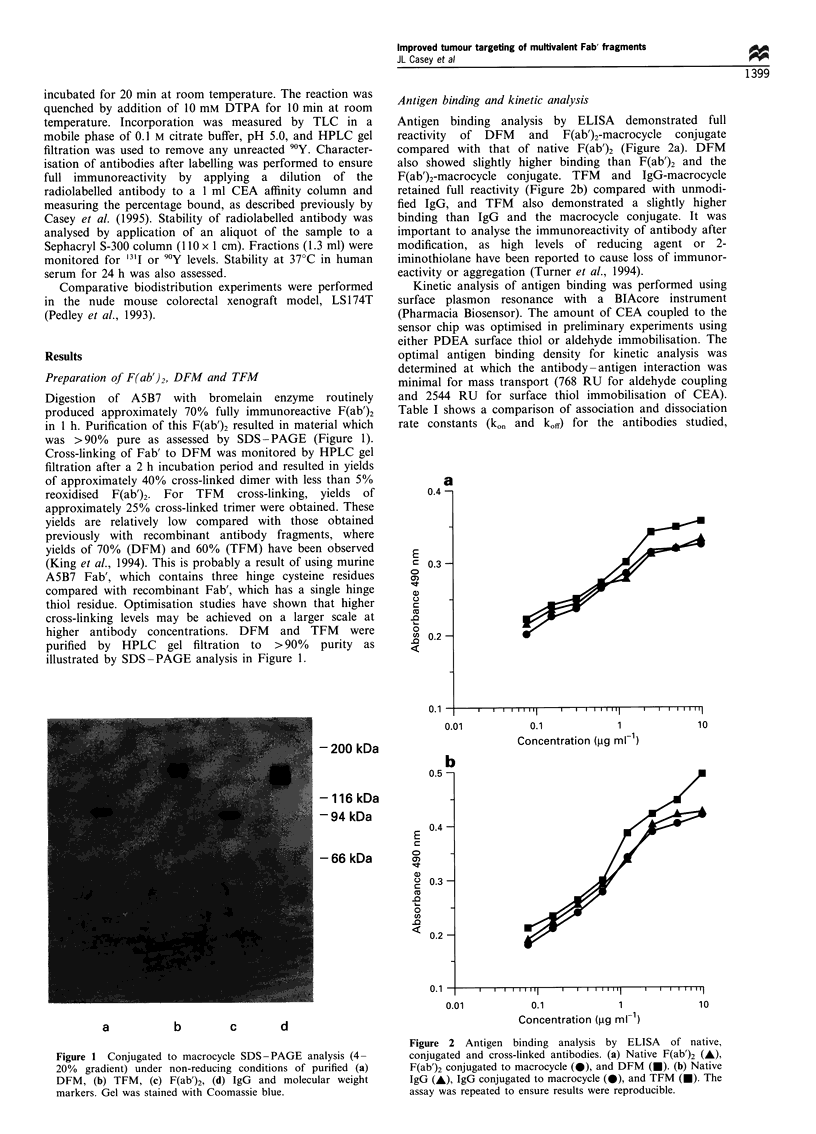
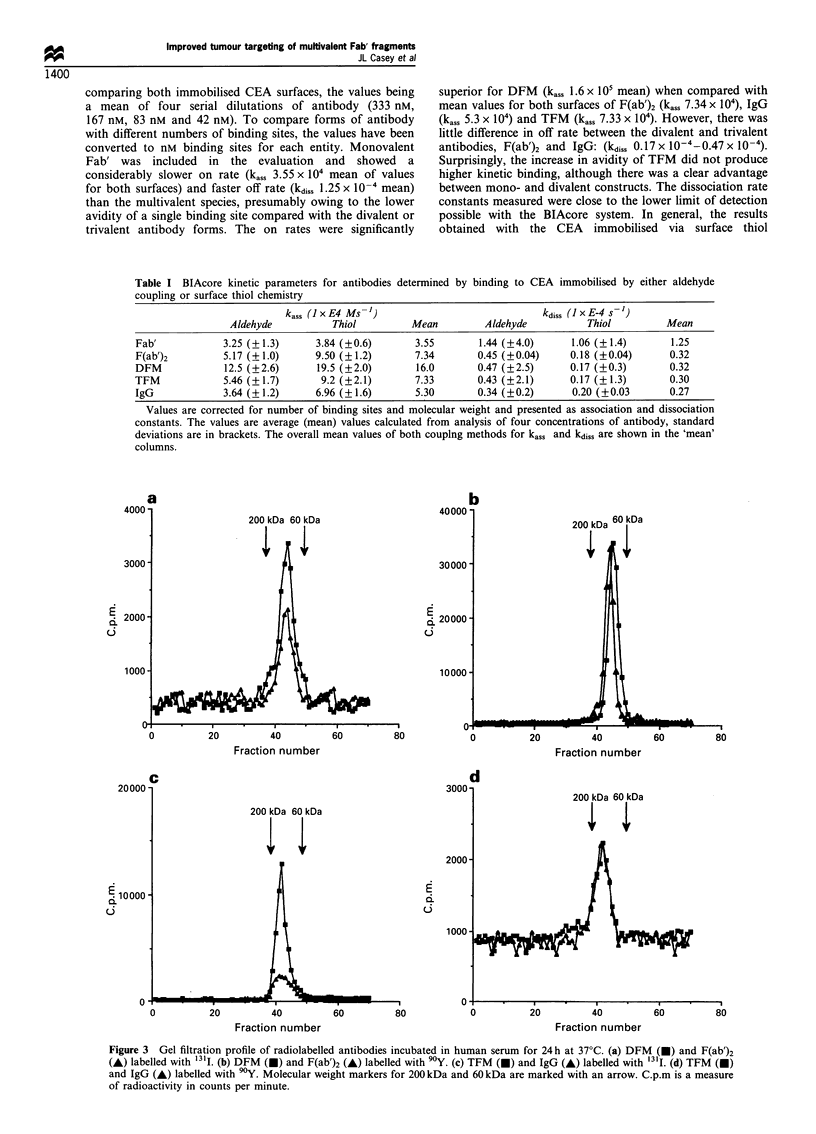
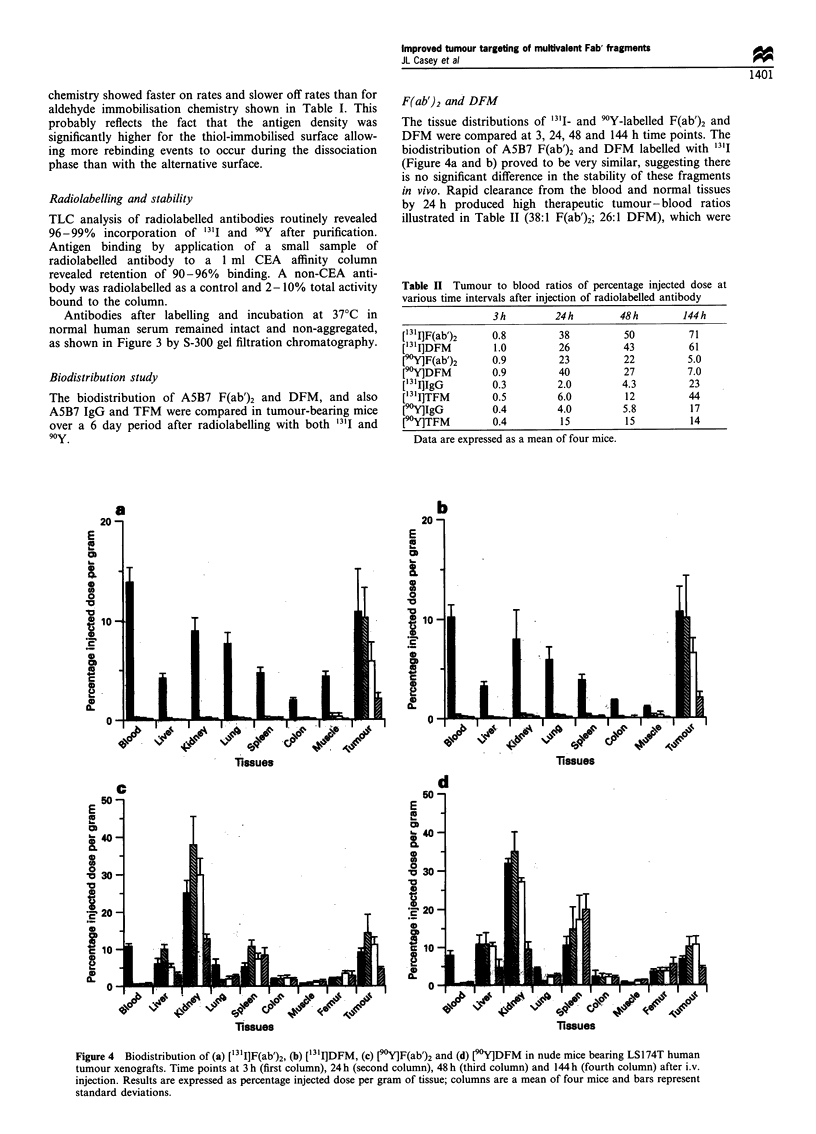
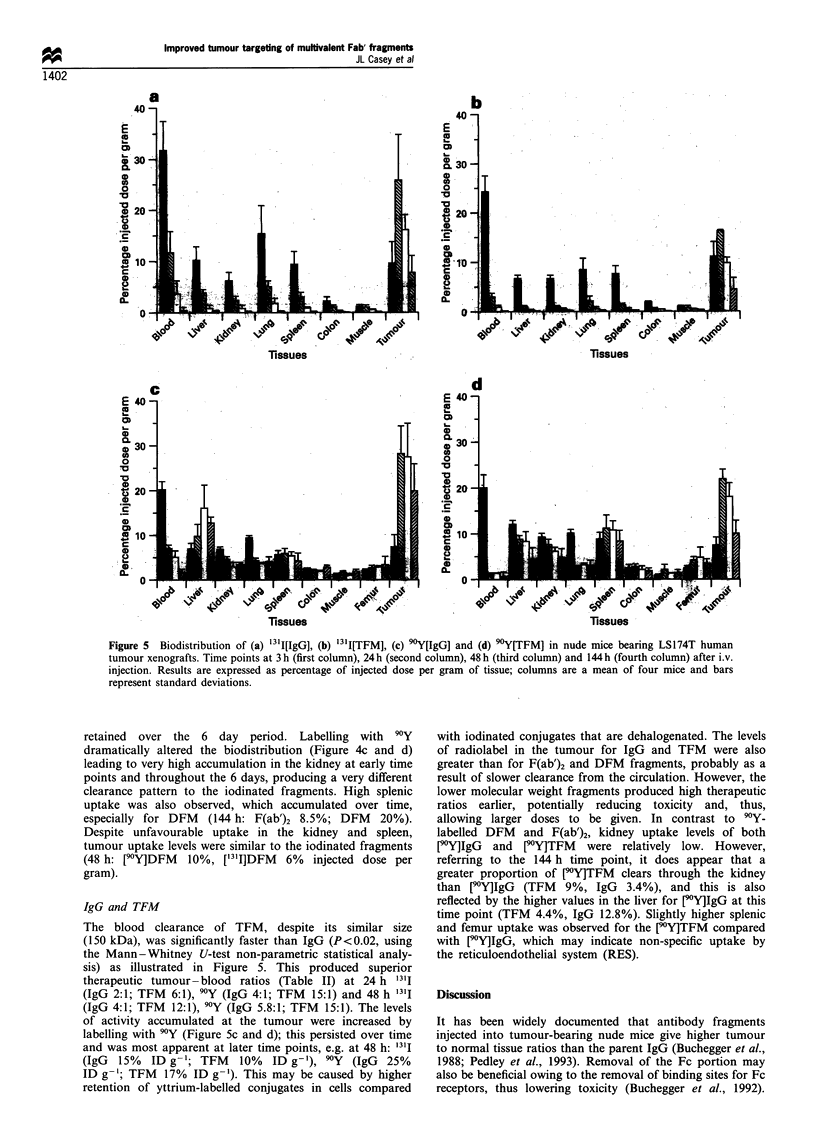



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R., Buxbaum S., Link J., Smith R., Venti C., Darsley M. Screening and kinetic analysis of recombinant anti-CEA antibody fragments. J Immunol Methods. 1995 Jun 14;183(1):119–125. doi: 10.1016/0022-1759(95)00039-d. [DOI] [PubMed] [Google Scholar]
- Andrew S. M., Perkins A. C., Pimm M. V., Baldwin R. W. A comparison of iodine and indium labelled anti CEA intact antibody, F(ab)2 and Fab fragments by imaging tumour xenografts. Eur J Nucl Med. 1988;13(11):598–604. doi: 10.1007/BF02574776. [DOI] [PubMed] [Google Scholar]
- Begent R. H., Pedley R. B. Antibody targeted therapy in cancer: comparison of murine and clinical studies. Cancer Treat Rev. 1990 Sep;17(2-3):373–378. doi: 10.1016/0305-7372(90)90071-m. [DOI] [PubMed] [Google Scholar]
- Boxer G. M., Begent R. H., Kelly A. M., Southall P. J., Blair S. B., Theodorou N. A., Dawson P. M., Ledermann J. A. Factors influencing variability of localisation of antibodies to carcinoembryonic antigen (CEA) in patients with colorectal carcinoma--implications for radioimmunotherapy. Br J Cancer. 1992 Jun;65(6):825–831. doi: 10.1038/bjc.1992.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. A., Comeau R. D., Jones P. L., Liberatore F. A., Neacy W. P., Sands H., Gallagher B. M. Pharmacokinetics of the monoclonal antibody B72.3 and its fragments labeled with either 125I or 111In. Cancer Res. 1987 Feb 15;47(4):1149–1154. [PubMed] [Google Scholar]
- Buchegger F., Pèlegrin A., Delaloye B., Bischof-Delaloye A., Mach J. P. Iodine-131-labeled MAb F(ab')2 fragments are more efficient and less toxic than intact anti-CEA antibodies in radioimmunotherapy of large human colon carcinoma grafted in nude mice. J Nucl Med. 1990 Jun;31(6):1035–1044. [PubMed] [Google Scholar]
- Buchegger F., Pèlegrin A., Hardman N., Heusser C., Lukas J., Dolci W., Mach J. P. Different behaviour of mouse-human chimeric antibody F(ab')2 fragments of IgG1, IgG2 and IgG4 sub-class in vivo. Int J Cancer. 1992 Feb 1;50(3):416–422. doi: 10.1002/ijc.2910500316. [DOI] [PubMed] [Google Scholar]
- Buchegger F., Vacca A., Carrel S., Schreyer M., Mach J. P. Radioimmunotherapy of human colon carcinoma by 131I-labelled monoclonal anti-CEA antibodies in a nude mouse model. Int J Cancer. 1988 Jan 15;41(1):127–134. doi: 10.1002/ijc.2910410123. [DOI] [PubMed] [Google Scholar]
- Carter P., Kelley R. F., Rodrigues M. L., Snedecor B., Covarrubias M., Velligan M. D., Wong W. L., Rowland A. M., Kotts C. E., Carver M. E. High level Escherichia coli expression and production of a bivalent humanized antibody fragment. Biotechnology (N Y) 1992 Feb;10(2):163–167. doi: 10.1038/nbt0292-163. [DOI] [PubMed] [Google Scholar]
- Casey J. L., Keep P. A., Chester K. A., Robson L., Hawkins R. E., Begent R. H. Purification of bacterially expressed single chain Fv antibodies for clinical applications using metal chelate chromatography. J Immunol Methods. 1995 Feb 13;179(1):105–116. doi: 10.1016/0022-1759(94)00278-5. [DOI] [PubMed] [Google Scholar]
- Chester K. A., Begent R. H., Robson L., Keep P., Pedley R. B., Boden J. A., Boxer G., Green A., Winter G., Cochet O. Phage libraries for generation of clinically useful antibodies. Lancet. 1994 Feb 19;343(8895):455–456. doi: 10.1016/s0140-6736(94)92695-6. [DOI] [PubMed] [Google Scholar]
- Covell D. G., Barbet J., Holton O. D., Black C. D., Parker R. J., Weinstein J. N. Pharmacokinetics of monoclonal immunoglobulin G1, F(ab')2, and Fab' in mice. Cancer Res. 1986 Aug;46(8):3969–3978. [PubMed] [Google Scholar]
- Cumber A. J., Ward E. S., Winter G., Parnell G. D., Wawrzynczak E. J. Comparative stabilities in vitro and in vivo of a recombinant mouse antibody FvCys fragment and a bisFvCys conjugate. J Immunol. 1992 Jul 1;149(1):120–126. [PubMed] [Google Scholar]
- Delaloye A. B., Delaloye B. Radiolabelled monoclonal antibodies in tumour imaging and therapy: out of fashion? Eur J Nucl Med. 1995 Jun;22(6):571–580. doi: 10.1007/BF00817285. [DOI] [PubMed] [Google Scholar]
- Glennie M. J., McBride H. M., Worth A. T., Stevenson G. T. Preparation and performance of bispecific F(ab' gamma)2 antibody containing thioether-linked Fab' gamma fragments. J Immunol. 1987 Oct 1;139(7):2367–2375. [PubMed] [Google Scholar]
- Goodwin D. A., Meares C. F., Watanabe N., McTigue M., Chaovapong W., Ransone C. M., Renn O., Greiner D. P., Kukis D. L., Kronenberger S. I. Pharmacokinetics of pretargeted monoclonal antibody 2D12.5 and 88Y-Janus-2-(p-nitrobenzyl)-1,4,7,10-tetraazacyclododecanetetraacetic acid (DOTA) in BALB/c mice with KHJJ mouse adenocarcinoma: a model for 90Y radioimmunotherapy. Cancer Res. 1994 Nov 15;54(22):5937–5946. [PubMed] [Google Scholar]
- Hird V., Verhoeyen M., Badley R. A., Price D., Snook D., Kosmas C., Gooden C., Bamias A., Meares C., Lavender J. P. Tumour localisation with a radioactively labelled reshaped human monoclonal antibody. Br J Cancer. 1991 Nov;64(5):911–914. doi: 10.1038/bjc.1991.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnatowich D. J., Childs R. L., Lanteigne D., Najafi A. The preparation of DTPA-coupled antibodies radiolabeled with metallic radionuclides: an improved method. J Immunol Methods. 1983 Dec 16;65(1-2):147–157. doi: 10.1016/0022-1759(83)90311-3. [DOI] [PubMed] [Google Scholar]
- Jurcic J. G., Scheinberg D. A. Recent developments in the radioimmunotherapy of cancer. Curr Opin Immunol. 1994 Oct;6(5):715–721. doi: 10.1016/0952-7915(94)90074-4. [DOI] [PubMed] [Google Scholar]
- King D. J., Antoniw P., Owens R. J., Adair J. R., Haines A. M., Farnsworth A. P., Finney H., Lawson A. D., Lyons A., Baker T. S. Preparation and preclinical evaluation of humanised A33 immunoconjugates for radioimmunotherapy. Br J Cancer. 1995 Dec;72(6):1364–1372. doi: 10.1038/bjc.1995.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D. J., Turner A., Farnsworth A. P., Adair J. R., Owens R. J., Pedley R. B., Baldock D., Proudfoot K. A., Lawson A. D., Beeley N. R. Improved tumor targeting with chemically cross-linked recombinant antibody fragments. Cancer Res. 1994 Dec 1;54(23):6176–6185. [PubMed] [Google Scholar]
- Lane D. M., Eagle K. F., Begent R. H., Hope-Stone L. D., Green A. J., Casey J. L., Keep P. A., Kelly A. M., Ledermann J. A., Glaser M. G. Radioimmunotherapy of metastatic colorectal tumours with iodine-131-labelled antibody to carcinoembryonic antigen: phase I/II study with comparative biodistribution of intact and F(ab')2 antibodies. Br J Cancer. 1994 Sep;70(3):521–525. doi: 10.1038/bjc.1994.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson S. M., Divgi C. R., Scott A. M. Overview of clinical radioimmunodetection of human tumors. Cancer. 1994 Feb 1;73(3 Suppl):832–835. doi: 10.1002/1097-0142(19940201)73:3+<832::aid-cncr2820731313>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Norrgren K., Strand S. E., Nilsson R., Lindgren L., Sjögren H. O. A general, extracorporeal immunoadsorption method to increase the tumor-to-normal tissue ratio in radioimmunoimaging and radioimmunotherapy. J Nucl Med. 1993 Mar;34(3):448–454. [PubMed] [Google Scholar]
- Pedley R. B., Boden J. A., Boden R., Dale R., Begent R. H. Comparative radioimmunotherapy using intact or F(ab')2 fragments of 131I anti-CEA antibody in a colonic xenograft model. Br J Cancer. 1993 Jul;68(1):69–73. doi: 10.1038/bjc.1993.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimm M. V., Andrew S. M., Baldwin R. W. Blood and tissue kinetics of radiolabelled anti-CEA monoclonal antibody and F(ab)2 and Fab fragments in nude mice with human tumour xenografts: implications for tumour imaging and radioimmunotherapy. Nucl Med Commun. 1989 Aug;10(8):585–593. doi: 10.1097/00006231-198908010-00007. [DOI] [PubMed] [Google Scholar]
- Press O. W., DeSantes K., Anderson S. K., Geissler F. Inhibition of catabolism of radiolabeled antibodies by tumor cells using lysosomotropic amines and carboxylic ionophores. Cancer Res. 1990 Feb 15;50(4):1243–1250. [PubMed] [Google Scholar]
- Quadri S. M., Lai J., Mohammadpour H., Vriesendorp H. M., Williams J. R. Assessment of radiolabeled stabilized F(ab')2 fragments of monoclonal antiferritin in nude mouse model. J Nucl Med. 1993 Dec;34(12):2152–2159. [PubMed] [Google Scholar]
- Schott M. E., Frazier K. A., Pollock D. K., Verbanac K. M. Preparation, characterization, and in vivo biodistribution properties of synthetically cross-linked multivalent antitumor antibody fragments. Bioconjug Chem. 1993 Mar-Apr;4(2):153–165. doi: 10.1021/bc00020a008. [DOI] [PubMed] [Google Scholar]
- Schott M. E., Milenic D. E., Yokota T., Whitlow M., Wood J. F., Fordyce W. A., Cheng R. C., Schlom J. Differential metabolic patterns of iodinated versus radiometal chelated anticarcinoma single-chain Fv molecules. Cancer Res. 1992 Nov 15;52(22):6413–6417. [PubMed] [Google Scholar]
- Sharkey R. M., Motta-Hennessy C., Pawlyk D., Siegel J. A., Goldenberg D. M. Biodistribution and radiation dose estimates for yttrium- and iodine-labeled monoclonal antibody IgG and fragments in nude mice bearing human colonic tumor xenografts. Cancer Res. 1990 Apr 15;50(8):2330–2336. [PubMed] [Google Scholar]
- Sumpio B. E., Hayslett J. P. Renal handling of proteins in normal and disease states. Q J Med. 1985 Oct;57(222):611–635. [PubMed] [Google Scholar]
- Turner A., King D. J., Farnsworth A. P., Rhind S. K., Pedley R. B., Boden J., Boden R., Millican T. A., Millar K., Boyce B. Comparative biodistributions of indium-111-labelled macrocycle chimeric B72.3 antibody conjugates in tumour-bearing mice. Br J Cancer. 1994 Jul;70(1):35–41. doi: 10.1038/bjc.1994.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutt A., Stevenson G. T., Glennie M. J. Trispecific F(ab')3 derivatives that use cooperative signaling via the TCR/CD3 complex and CD2 to activate and redirect resting cytotoxic T cells. J Immunol. 1991 Jul 1;147(1):60–69. [PubMed] [Google Scholar]
- Verhaar M. J., Keep P. A., Hawkins R. E., Robson L., Casey J. L., Pedley B., Boden J. A., Begent R. H., Chester K. A. Technetium-99m radiolabeling using a phage-derived single-chain Fv with a C-terminal cysteine. J Nucl Med. 1996 May;37(5):868–872. [PubMed] [Google Scholar]
- Waldmann T. A. Monoclonal antibodies in diagnosis and therapy. Science. 1991 Jun 21;252(5013):1657–1662. doi: 10.1126/science.2047874. [DOI] [PubMed] [Google Scholar]
- Werlen R. C., Lankinen M., Offord R. E., Schubiger P. A., Smith A., Rose K. Preparation of a trivalent antigen-binding construct using polyoxime chemistry: improved biodistribution and potential for therapeutic application. Cancer Res. 1996 Feb 15;56(4):809–815. [PubMed] [Google Scholar]
- Yokota T., Milenic D. E., Whitlow M., Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992 Jun 15;52(12):3402–3408. [PubMed] [Google Scholar]
- Yorke E. D., Beaumier P. L., Wessels B. W., Fritzberg A. R., Morgan A. C., Jr Optimal antibody-radionuclide combinations for clinical radioimmunotherapy: a predictive model based on mouse pharmacokinetics. Int J Rad Appl Instrum B. 1991;18(8):827–835. doi: 10.1016/0883-2897(91)90090-8. [DOI] [PubMed] [Google Scholar]




