Abstract
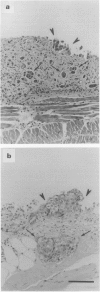
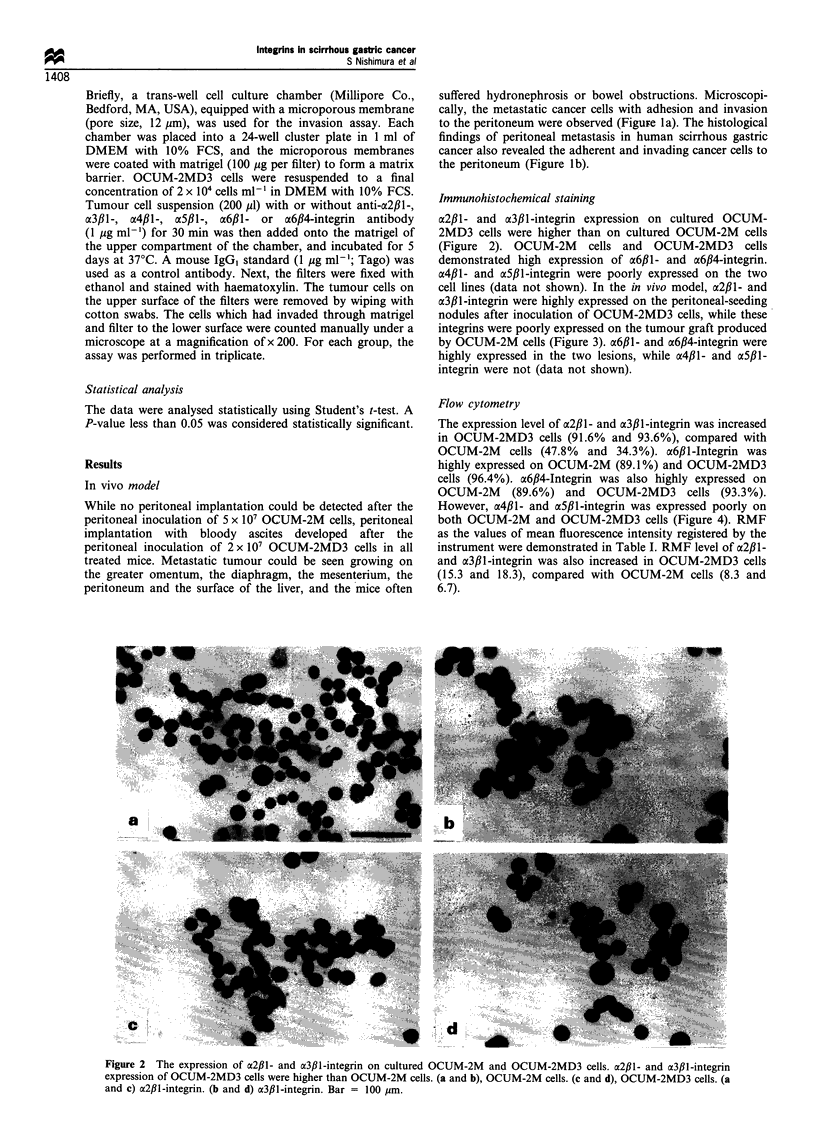
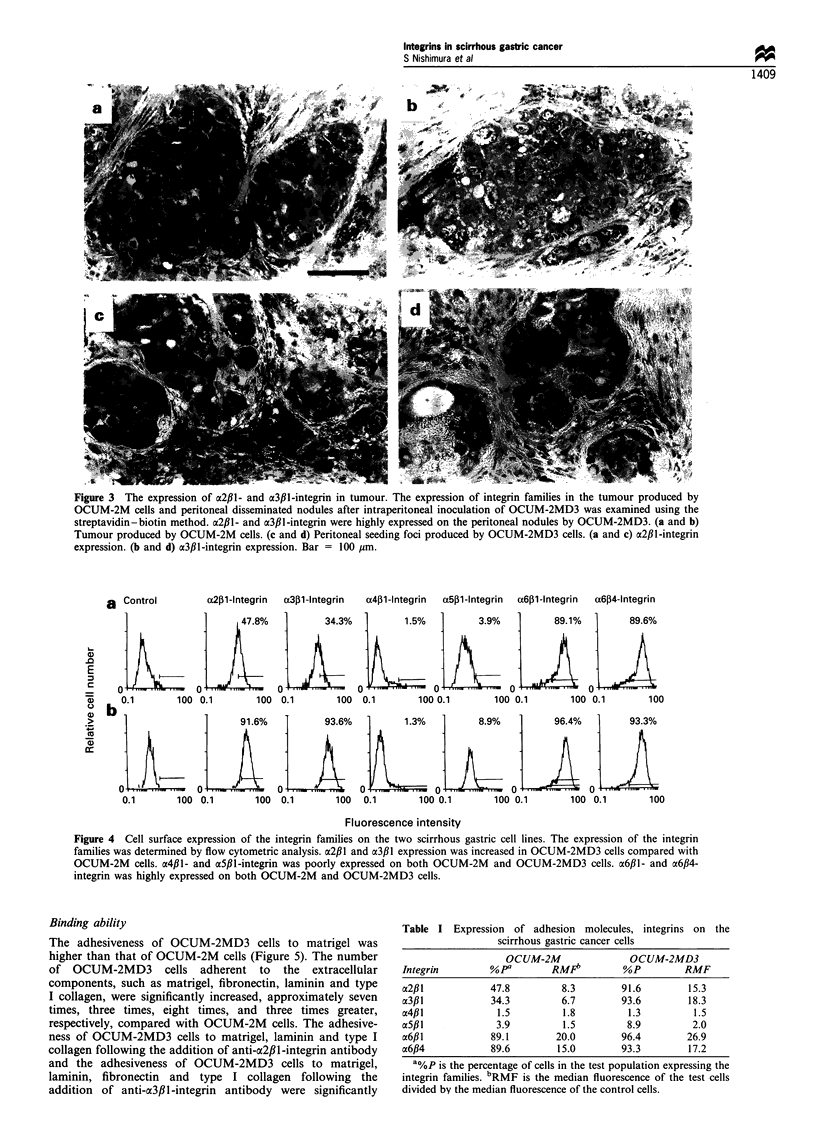
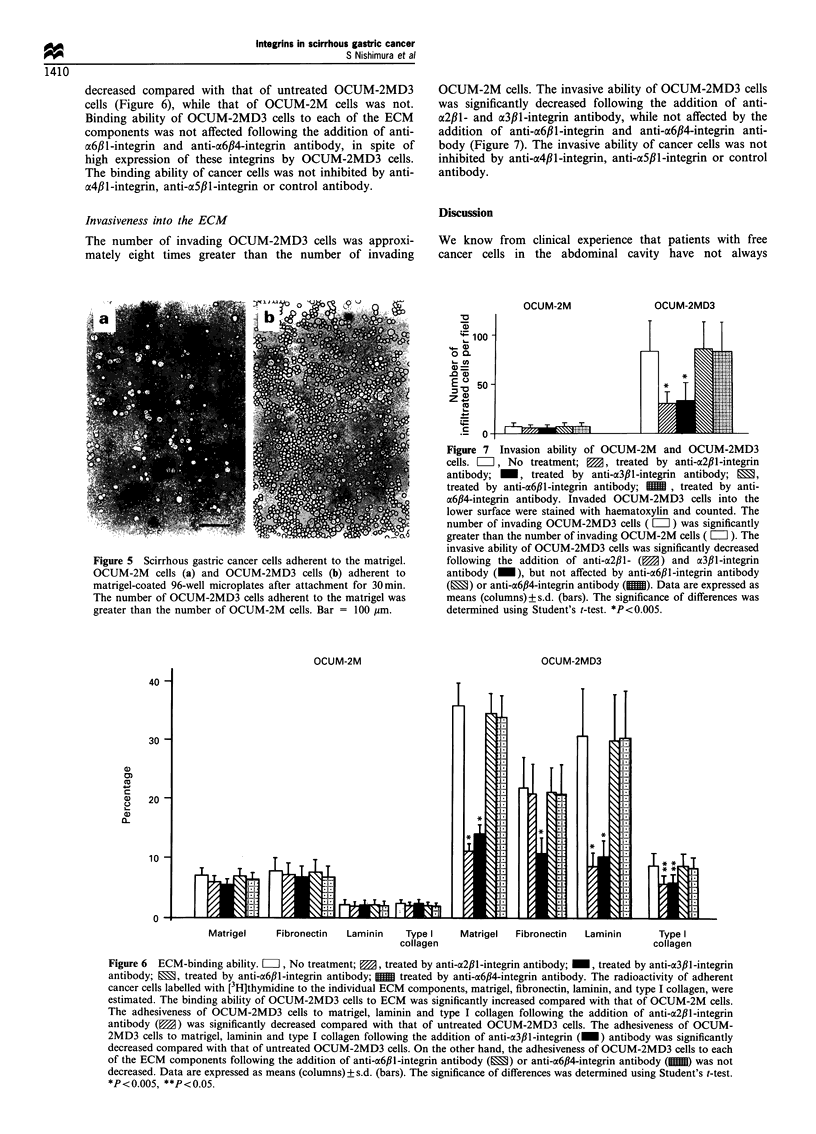
We established a highly peritoneal-seeding cell line, OCUM-2MD3, from a poorly peritoneal-seeding cell line, OCUM-2M, of human scirrhous gastric carcinoma. The intraperitoneal inoculation of OCUM-2MD3 cells produced peritoneal dissemination in nude mice, whereas that of OCUM-2M cells did not. We then investigated the correlation between seeding potential and adhesion molecule beta 1-integrins or alpha 6 beta 4-integrin. alpha 2 beta 1- and alpha 3 beta 1-integrin expression on OCUM-2MD3 cells (91.6% and 93.6%) was increased compared with that of OCUM-2M cells (47.8% and 34.3%) by flow cytometric analysis, and the expression level of the other integrins was not different between the two cell lines. The binding ability of OCUM-2MD3 cells to matrigel, fibronectin, laminin and type I collagen was significantly increased, approximately seven times, three times, eight times, and three times greater than that of OCUM-2M cells respectively. The invasiveness of OCUM-2MD3 cells was also significantly increased 8-fold over OCUM-2M cells. The binding and invasive ability of OCUM-2MD3 cells was significantly decreased following the addition of anti-alpha 2 beta 1- and alpha 3 beta 1-integrin antibody, but not by anti-alpha 6 beta 1- and alpha 6 beta 4-integrin antibody. These results suggest that adhesiveness and invasiveness in peritoneal implantation of scirrhous gastric carcinoma might be closely associated with alpha 2 beta 1- and alpha 3 beta 1-integrin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Iwamoto Y., Kleinman H. K., Martin G. R., Aaronson S. A., Kozlowski J. M., McEwan R. N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987 Jun 15;47(12):3239–3245. [PubMed] [Google Scholar]
- BIRBECK M. S., WHEATLEY D. N. AN ELECTRON MICROSCOPIC STUDY OF THE INVASION OF ASCITES TUMOR CELLS INTO THE ABDOMINAL WALL. Cancer Res. 1965 May;25:490–497. [PubMed] [Google Scholar]
- Buck R. C. Walker 256 tumor implantation in normal and injured peritoneum studied by electron microscopy, scanning electron microscopy, and autoradiography. Cancer Res. 1973 Dec;33(12):3181–3188. [PubMed] [Google Scholar]
- Chan B. M., Matsuura N., Takada Y., Zetter B. R., Hemler M. E. In vitro and in vivo consequences of VLA-2 expression on rhabdomyosarcoma cells. Science. 1991 Mar 29;251(5001):1600–1602. doi: 10.1126/science.2011740. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Urry L. A., Hemler M. E. Receptor functions for the integrin VLA-3: fibronectin, collagen, and laminin binding are differentially influenced by Arg-Gly-Asp peptide and by divalent cations. J Cell Biol. 1991 Jan;112(1):169–181. doi: 10.1083/jcb.112.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990 Oct 1;50(19):6130–6138. [PubMed] [Google Scholar]
- Fujita S., Suzuki H., Kinoshita M., Hirohashi S. Inhibition of cell attachment, invasion and metastasis of human carcinoma cells by anti-integrin beta 1 subunit antibody. Jpn J Cancer Res. 1992 Dec;83(12):1317–1326. doi: 10.1111/j.1349-7006.1992.tb02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Hall D. E., Reichardt L. F., Crowley E., Holley B., Moezzi H., Sonnenberg A., Damsky C. H. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990 Jun;110(6):2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart I. R. 'Seed and soil' revisited: mechanisms of site-specific metastasis. Cancer Metastasis Rev. 1982;1(1):5–16. doi: 10.1007/BF00049477. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kaneshima S., Kudo H., Kosaka H., Iitsuka Y., Kimachi H. A scanning electron microscopic study on implantation of Ehrlich ascites tumor cells in the peritoneal layer. Yonago Acta Med. 1976 Aug;20(2):101–107. [PubMed] [Google Scholar]
- Kimura A., Koga S., Kudoh H., Iitsuka Y. Peritoneal mesothelial cell injury factors in rat cancerous ascites. Cancer Res. 1985 Sep;45(9):4330–4333. [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Languino L. R., Gehlsen K. R., Wayner E., Carter W. G., Engvall E., Ruoslahti E. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. J Cell Biol. 1989 Nov;109(5):2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. C., Lotz M. M., Steele G. D., Jr, Mercurio A. M. The integrin alpha 6 beta 4 is a laminin receptor. J Cell Biol. 1992 May;117(3):671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Mould A. P., Komoriya A., Yamada K. M., Humphries M. J. The CS5 peptide is a second site in the IIICS region of fibronectin recognized by the integrin alpha 4 beta 1. Inhibition of alpha 4 beta 1 function by RGD peptide homologues. J Biol Chem. 1991 Feb 25;266(6):3579–3585. [PubMed] [Google Scholar]
- Ruoslahti E., Giancotti F. G. Integrins and tumor cell dissemination. Cancer Cells. 1989 Dec;1(4):119–126. [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Saga S., Chen W. T., Yamada K. M. Enhanced fibronectin receptor expression in Rous sarcoma virus-induced tumors. Cancer Res. 1988 Oct 1;48(19):5510–5513. [PubMed] [Google Scholar]
- Sowa M., Kato Y., Nishimura M., Yoshino H., Kubo T., Umeyama K. Clinico-histochemical studies on type 4 carcinoma of the stomach--with special reference to mucopolysaccharides and sialic acid in tumor tissue. Jpn J Surg. 1989 Mar;19(2):153–162. doi: 10.1007/BF02471579. [DOI] [PubMed] [Google Scholar]
- Sriramarao P., Steffner P., Gehlsen K. R. Biochemical evidence for a homophilic interaction of the alpha 3 beta 1 integrin. J Biol Chem. 1993 Oct 15;268(29):22036–22041. [PubMed] [Google Scholar]
- Yashiro M., Chung Y. S., Nishimura S., Inoue T., Sowa M. Establishment of two new scirrhous gastric cancer cell lines: analysis of factors associated with disseminated metastasis. Br J Cancer. 1995 Nov;72(5):1200–1210. doi: 10.1038/bjc.1995.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro M., Chung Y. S., Nishimura S., Inoue T., Sowa M. Peritoneal metastatic model for human scirrhous gastric carcinoma in nude mice. Clin Exp Metastasis. 1996 Jan;14(1):43–54. doi: 10.1007/BF00157685. [DOI] [PubMed] [Google Scholar]
- Yashiro M., Chung Y. S., Sowa M. Role of orthotopic fibroblasts in the development of scirrhous gastric carcinoma. Jpn J Cancer Res. 1994 Sep;85(9):883–886. doi: 10.1111/j.1349-7006.1994.tb02963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]