Abstract
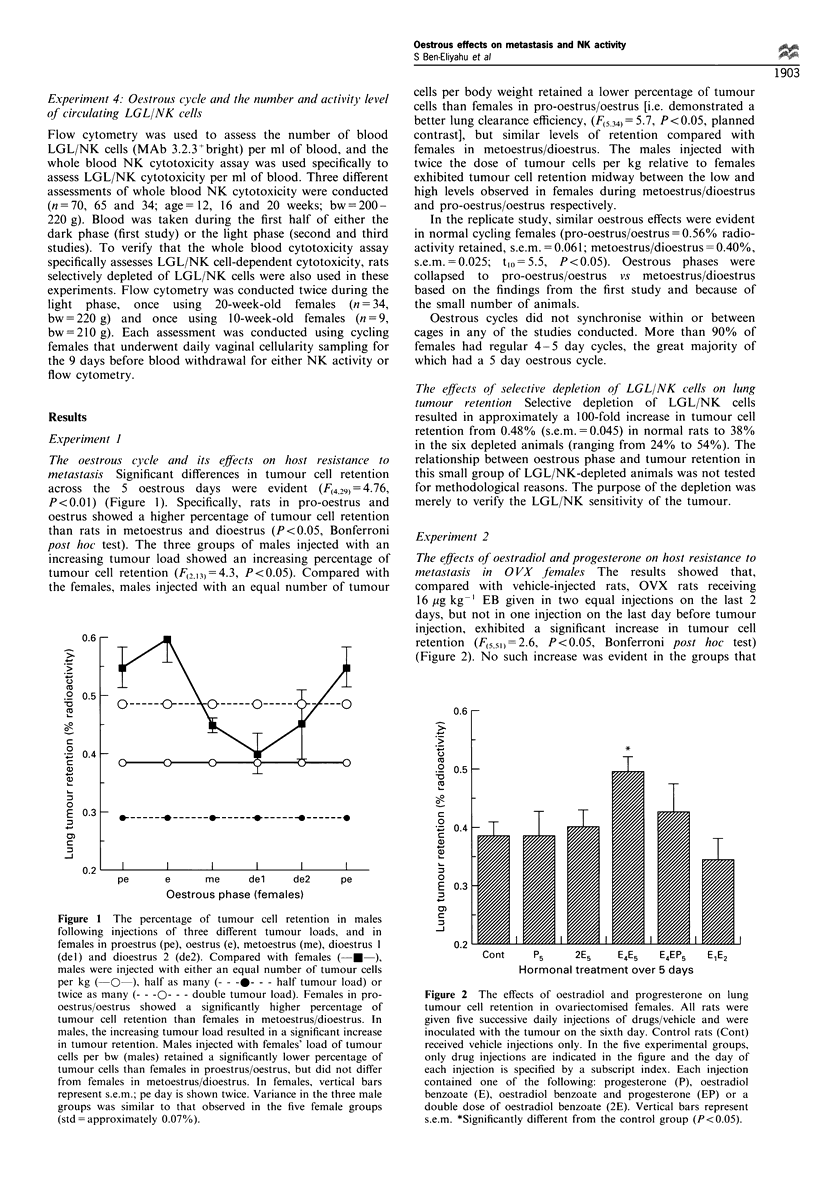
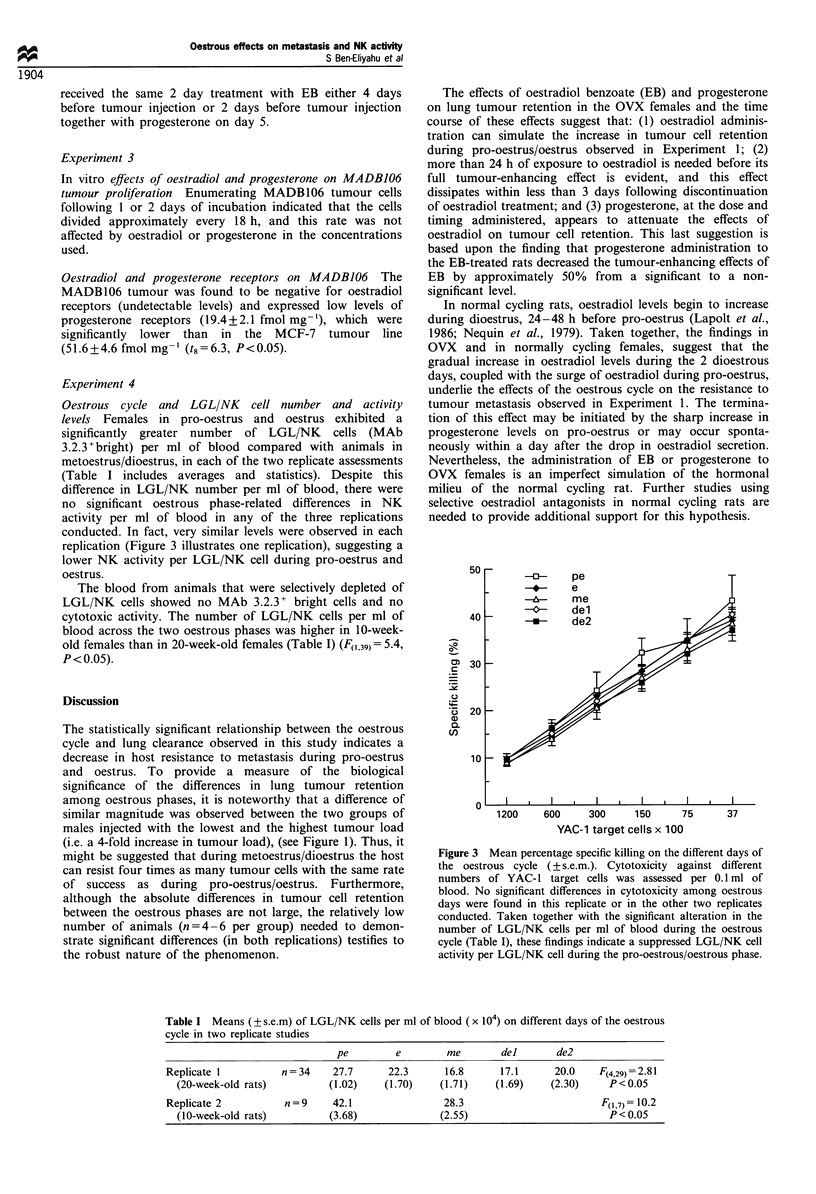
It has been suggested that tumour development and immunocompetence are affected by the menstrual and the oestrous cycle, and sex hormones have been shown to modulate lymphokine production, neuroendocrine activity and immunity. In this study, we assessed natural killer cell activity and host susceptibility to metastasis during the oestrous cycle in the Fischer 344 inbred rat strain. Females were inoculated intravenously with MADB106 tumour cells, a syngeneic mammary adenocarcinoma cell line that metastasises only to the lungs. The susceptibility to metastatic development of this tumour was found to be significantly higher during pro-oestrus and oestrus than during metoestrus and dioestrus. Two days of exposure to oestradiol benzoate caused similar effects in ovariectomised females, and a single administration of progesterone reduced this effect of oestradiol to a statistically non-significant level. The tumour was found to be negative for oestradiol receptors, and its in vitro proliferation rate was not affected by oestradiol or progesterone, suggesting that the effects of sex hormones on the metastatic process are not attributable to a direct effect on tumour cells. Because the metastatic process of MADB106 tumour cells is known, and confirmed here, to be highly controlled by large granular lymphocyte/natural killer (LGL/NK) cell activity, we assessed their role in mediating the effects of the oestrous cycle. The number and activity levels of circulating blood LG/NK cells (NKR-PI+ bright) were studied. Findings indicated oestrous-dependent alterations in the number of LGL/NK cells and suggested a diminished NK activity per LGL/NK cell during pro-oestrus/ oestrus, the same phases that were characterised by higher susceptibility to metastatic development. These findings provide the first empirical evidence for a causal relationship between a short-term exposure to elevated oestradiol/low progesterone levels and decreased resistance to tumour metastasis, and it is hypothesised that an alteration in LGL/NK cell activity underlies these effects. Homologies and relevance to clinical phenomena are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badwe R. A., Gregory W. M., Chaudary M. A., Richards M. A., Bentley A. E., Rubens R. D., Fentiman I. S. Timing of surgery during menstrual cycle and survival of premenopausal women with operable breast cancer. Lancet. 1991 May 25;337(8752):1261–1264. doi: 10.1016/0140-6736(91)92927-t. [DOI] [PubMed] [Google Scholar]
- Baker D. A., Salvatore W., Milch P. O. Effect of low-dose oral contraceptives on natural killer cell activity. Contraception. 1989 Jan;39(1):119–124. doi: 10.1016/0010-7824(89)90020-6. [DOI] [PubMed] [Google Scholar]
- Baral E., Kwok S., Berczi I. The influence of estradiol and tamoxifen on the mixed lymphocyte reaction in rats. Immunopharmacology. 1991 May-Jun;21(3):191–197. doi: 10.1016/0162-3109(91)90024-s. [DOI] [PubMed] [Google Scholar]
- Barlozzari T., Leonhardt J., Wiltrout R. H., Herberman R. B., Reynolds C. W. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. J Immunol. 1985 Apr;134(4):2783–2789. [PubMed] [Google Scholar]
- Barlozzari T., Reynolds C. W., Herberman R. B. In vivo role of natural killer cells: involvement of large granular lymphocytes in the clearance of tumor cells in anti-asialo GM1-treated rats. J Immunol. 1983 Aug;131(2):1024–1027. [PubMed] [Google Scholar]
- Ben-Eliyahu S., Page G. G., Yirmiya R., Taylor A. N. Acute alcohol intoxication suppresses natural killer cell activity and promotes tumor metastasis. Nat Med. 1996 Apr;2(4):457–460. doi: 10.1038/nm0496-457. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S., Yirmiya R., Liebeskind J. C., Taylor A. N., Gale R. P. Stress increases metastatic spread of a mammary tumor in rats: evidence for mediation by the immune system. Brain Behav Immun. 1991 Jun;5(2):193–205. doi: 10.1016/0889-1591(91)90016-4. [DOI] [PubMed] [Google Scholar]
- Callewaert D. M., Moudgil V. K., Radcliff G., Waite R. Hormone specific regulation of natural killer cells by cortisol. Direct inactivation of the cytotoxic function of cloned human NK cells without an effect on cellular proliferation. FEBS Lett. 1991 Jul 8;285(1):108–110. doi: 10.1016/0014-5793(91)80736-m. [DOI] [PubMed] [Google Scholar]
- Chambers W. H., Brumfield A. M., Hanley-Yanez K., Lakomy R., Herberman R. B., McCaslin D. C., Olszowy M. W., McCoy J. P., Jr Functional heterogeneity between NKR-P1bright/Lycopersicon esculentum lectin (L.E.)bright and NKR-P1bright/L.E.dim subpopulations of rat natural killer cells. J Immunol. 1992 Jun 1;148(11):3658–3665. [PubMed] [Google Scholar]
- Chambers W. H., Vujanovic N. L., DeLeo A. B., Olszowy M. W., Herberman R. B., Hiserodt J. C. Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. J Exp Med. 1989 Apr 1;169(4):1373–1389. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson N. E., Abeloff M. D. Menstrual effects on surgical treatment for breast cancer. Cancer Treat Rev. 1993 Apr;19(2):105–112. doi: 10.1016/0305-7372(93)90029-q. [DOI] [PubMed] [Google Scholar]
- Gabrilovac J., Zadjelović J., Osmak M., Suchanek E., Zupanović Z., Boranić M. NK cell activity and estrogen hormone levels during normal human pregnancy. Gynecol Obstet Invest. 1988;25(3):165–172. doi: 10.1159/000293766. [DOI] [PubMed] [Google Scholar]
- Geier A., Haimsohn M., Lunenfeld B. Evidence for the origin of the unoccupied oestrogen receptor in nuclei of a human breast-cancer cell line (MCF-7). Biochem J. 1982 Mar 15;202(3):687–691. doi: 10.1042/bj2020687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik E., Wiltrout R. H., Okumura K., Habu S., Herberman R. B. Role of NK cells in the control of metastatic spread and growth of tumor cells in mice. Int J Cancer. 1982 Jul 15;30(1):107–112. doi: 10.1002/ijc.2910300118. [DOI] [PubMed] [Google Scholar]
- Gruber S. A., Hoffman R. A., Sothern R. B., Lakatua D., Carlson A., Simmons R. L., Hrushesky W. J. Splenocyte natural killer cell activity and metastatic potential are inversely dependent on estrous stage. Surgery. 1988 Aug;104(2):398–403. [PubMed] [Google Scholar]
- Hanna N., Schneider M. Enhancement of tumor metastasis and suppression of natural killer cell activity by beta-estradiol treatment. J Immunol. 1983 Feb;130(2):974–980. [PubMed] [Google Scholar]
- Hanna N. The role of natural killer cells in the control of tumor growth and metastasis. Biochim Biophys Acta. 1985;780(3):213–226. doi: 10.1016/0304-419x(85)90004-6. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Hou J., Zheng W. F. Effect of sex hormones on NK and ADCC activity of mice. Int J Immunopharmacol. 1988;10(1):15–22. doi: 10.1016/0192-0561(88)90145-2. [DOI] [PubMed] [Google Scholar]
- Hrushesky W. J., Bluming A. Z., Gruber S. A., Sothern R. B. Menstrual influence on surgical cure of breast cancer. Lancet. 1989 Oct 21;2(8669):949–952. doi: 10.1016/s0140-6736(89)90956-2. [DOI] [PubMed] [Google Scholar]
- Hrushesky W. J., Gruber S. A., Sothern R. B., Hoffman R. A., Lakatua D., Carlson A., Cerra F., Simmons R. L. Natural killer cell activity: age, estrous- and circadian-stage dependence and inverse correlation with metastatic potential. J Natl Cancer Inst. 1988 Oct 5;80(15):1232–1237. doi: 10.1093/jnci/80.15.1232. [DOI] [PubMed] [Google Scholar]
- Jaso-Friedmann L., Leary J. H., 3rd, St John A. L., Harris D. T., Koren H. S., Evans D. L. Detection of function-associated molecules on rat NK cells and their role in target cell lysis. Cell Immunol. 1992 Apr 15;141(1):131–147. doi: 10.1016/0008-8749(92)90133-a. [DOI] [PubMed] [Google Scholar]
- Lapolt P. S., Matt D. W., Judd H. L., Lu J. K. The relation of ovarian steroid levels in young female rats to subsequent estrous cyclicity and reproductive function during aging. Biol Reprod. 1986 Dec;35(5):1131–1139. doi: 10.1095/biolreprod35.5.1131. [DOI] [PubMed] [Google Scholar]
- Levy S., Herberman R., Lippman M., d'Angelo T. Correlation of stress factors with sustained depression of natural killer cell activity and predicted prognosis in patients with breast cancer. J Clin Oncol. 1987 Mar;5(3):348–353. doi: 10.1200/JCO.1987.5.3.348. [DOI] [PubMed] [Google Scholar]
- Lynch E. A., Dinarello C. A., Cannon J. G. Gender differences in IL-1 alpha, IL-1 beta, and IL-1 receptor antagonist secretion from mononuclear cells and urinary excretion. J Immunol. 1994 Jul 1;153(1):300–306. [PubMed] [Google Scholar]
- Matt D. W., LaPolt P. S., Judd H. L., Lu J. K. Estrogen exposure affects the post-ovariectomy increases in both LH and FSH release in female rats. Neuroendocrinology. 1986;42(1):21–27. doi: 10.1159/000124243. [DOI] [PubMed] [Google Scholar]
- Nappi R. E., Guo A. L., Petraglia F., Bonati M. E., Criscuolo M., Ficarra G., Zara C., Genazzani A. R. Pituitary and ovarian interleukin-1 alpha content changes according to estrous cycle and acute stress exposure. Gynecol Endocrinol. 1994 Dec;8(4):259–264. doi: 10.3109/09513599409023630. [DOI] [PubMed] [Google Scholar]
- Nequin L. G., Alvarez J., Schwartz N. B. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol Reprod. 1979 Apr;20(3):659–670. doi: 10.1095/biolreprod20.3.659. [DOI] [PubMed] [Google Scholar]
- Oldham R. K. Natural killer cells: history, relevance, and clinical applications. Nat Immun Cell Growth Regul. 1990;9(5):297–312. [PubMed] [Google Scholar]
- Page G. G., Ben-Eliyahu S., Liebeskind J. C. The role of LGL/NK cells in surgery-induced promotion of metastasis and its attenuation by morphine. Brain Behav Immun. 1994 Sep;8(3):241–250. doi: 10.1006/brbi.1994.1022. [DOI] [PubMed] [Google Scholar]
- Page G. G., Ben-Eliyahu S., Taylor A. N. The development of sexual dimorphism in natural killer cell activity and resistance to tumor metastasis in the Fischer 344 rat. J Neuroimmunol. 1995 Dec;63(1):69–77. doi: 10.1016/0165-5728(95)00132-8. [DOI] [PubMed] [Google Scholar]
- Page G. G., Ben-Eliyahu S., Yirmiya R., Liebeskind J. C. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993 Jul;54(1):21–28. doi: 10.1016/0304-3959(93)90095-7. [DOI] [PubMed] [Google Scholar]
- Polan M. L., Daniele A., Kuo A. Gonadal steroids modulate human monocyte interleukin-1 (IL-1) activity. Fertil Steril. 1988 Jun;49(6):964–968. [PubMed] [Google Scholar]
- Polan M. L., Kuo A., Loukides J., Bottomly K. Cultured human luteal peripheral monocytes secrete increased levels of interleukin-1. J Clin Endocrinol Metab. 1990 Feb;70(2):480–484. doi: 10.1210/jcem-70-2-480. [DOI] [PubMed] [Google Scholar]
- Polan M. L., Loukides J. A., Honig J. Interleukin-1 in human ovarian cells and in peripheral blood monocytes increases during the luteal phase: evidence for a midcycle surge in the human. Am J Obstet Gynecol. 1994 Apr;170(4):1000–1007. doi: 10.1016/s0002-9378(94)70093-1. [DOI] [PubMed] [Google Scholar]
- Polan M. L., Loukides J., Nelson P., Carding S., Diamond M., Walsh A., Bottomly K. Progesterone and estradiol modulate interleukin-1 beta messenger ribonucleic acid levels in cultured human peripheral monocytes. J Clin Endocrinol Metab. 1989 Dec;69(6):1200–1206. doi: 10.1210/jcem-69-6-1200. [DOI] [PubMed] [Google Scholar]
- Saad Z., Bramwell V., Duff J., Girotti M., Jory T., Heathcote G., Turnbull I., Garcia B., Stitt L. Timing of surgery in relation to the menstrual cycle in premenopausal women with operable breast cancer. Br J Surg. 1994 Feb;81(2):217–220. doi: 10.1002/bjs.1800810219. [DOI] [PubMed] [Google Scholar]
- Schantz S. P., Brown B. W., Lira E., Taylor D. L., Beddingfield N. Evidence for the role of natural immunity in the control of metastatic spread of head and neck cancer. Cancer Immunol Immunother. 1987;25(2):141–148. doi: 10.1007/BF00199955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screpanti I., Santoni A., Gulino A., Herberman R. B., Frati L. Estrogen and antiestrogen modulation of the levels of mouse natural killer activity and large granular lymphocytes. Cell Immunol. 1987 May;106(2):191–202. doi: 10.1016/0008-8749(87)90163-8. [DOI] [PubMed] [Google Scholar]
- Seaman W. E., Gindhart T. D. Effect of estrogen on natural killer cells. Arthritis Rheum. 1979 Nov;22(11):1234–1240. doi: 10.1002/art.1780221110. [DOI] [PubMed] [Google Scholar]
- Senie R. T., Rosen P. P., Rhodes P., Lesser M. L. Timing of breast cancer excision during the menstrual cycle influences duration of disease-free survival. Ann Intern Med. 1991 Sep 1;115(5):337–342. doi: 10.7326/0003-4819-115-5-337. [DOI] [PubMed] [Google Scholar]
- Simón C., Piquette G. N., Frances A., Westphal L. M., Heinrichs W. L., Polan M. L. Interleukin-1 type I receptor messenger ribonucleic acid expression in human endometrium throughout the menstrual cycle. Fertil Steril. 1993 Apr;59(4):791–796. doi: 10.1016/s0015-0282(16)55861-0. [DOI] [PubMed] [Google Scholar]
- Sulke A. N., Jones D. B., Wood P. J. Variation in natural killer activity in peripheral blood during the menstrual cycle. Br Med J (Clin Res Ed) 1985 Mar 23;290(6472):884–886. doi: 10.1136/bmj.290.6472.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi U., Luini A., Mariani L., Del Vecchio M., Alvez D., Andreoli C., Giacobone A., Merson M., Pacetti G., Raselli R. Effect of menstrual phase on surgical treatment of breast cancer. Lancet. 1994 Jun 18;343(8912):1545–1547. doi: 10.1016/s0140-6736(94)92942-4. [DOI] [PubMed] [Google Scholar]
- Viau V., Meaney M. J. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991 Nov;129(5):2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Vidaller A., Guadarrama F., Llorente L., Méndez J. B., Larrea F., Villa A. R., Alarcón-Segovia D. Hyperprolactinemia inhibits natural killer (NK) cell function in vivo and its bromocriptine treatment not only corrects it but makes it more efficient. J Clin Immunol. 1992 May;12(3):210–215. doi: 10.1007/BF00918091. [DOI] [PubMed] [Google Scholar]
- White D., Jones D. B., Cooke T., Kirkham N. Natural killer (NK) activity in peripheral blood lymphocytes of patients with benign and malignant breast disease. Br J Cancer. 1982 Oct;46(4):611–616. doi: 10.1038/bjc.1982.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltrout R. H., Herberman R. B., Zhang S. R., Chirigos M. A., Ortaldo J. R., Green K. M., Jr, Talmadge J. E. Role of organ-associated NK cells in decreased formation of experimental metastases in lung and liver. J Immunol. 1985 Jun;134(6):4267–4275. [PubMed] [Google Scholar]
- Yirmiya R., Ben-Eliyahu S., Gale R. P., Shavit Y., Liebeskind J. C., Taylor A. N. Ethanol increases tumor progression in rats: possible involvement of natural killer cells. Brain Behav Immun. 1992 Mar;6(1):74–86. doi: 10.1016/0889-1591(92)90061-r. [DOI] [PubMed] [Google Scholar]
- van den Brink M. R., Hunt L. E., Hiserodt J. C. In vivo treatment with monoclonal antibody 3.2.3 selectively eliminates natural killer cells in rats. J Exp Med. 1990 Jan 1;171(1):197–210. doi: 10.1084/jem.171.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]


