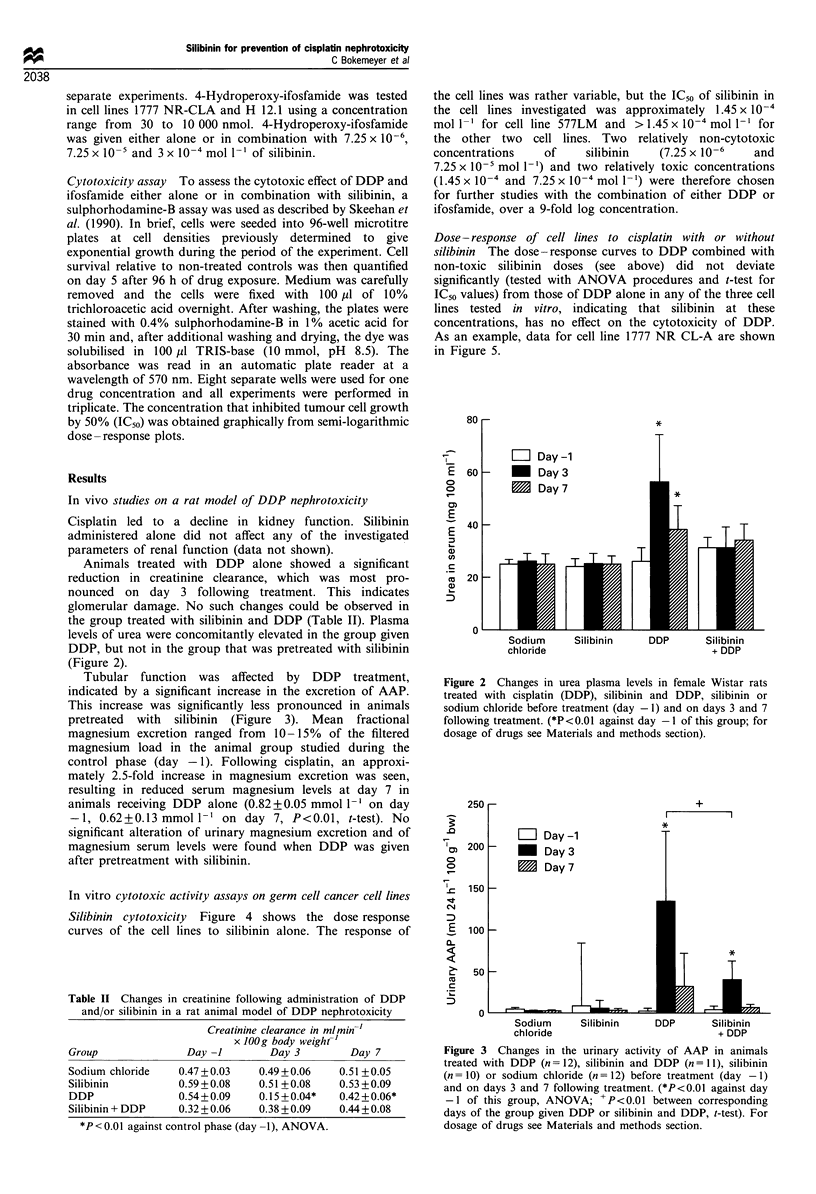
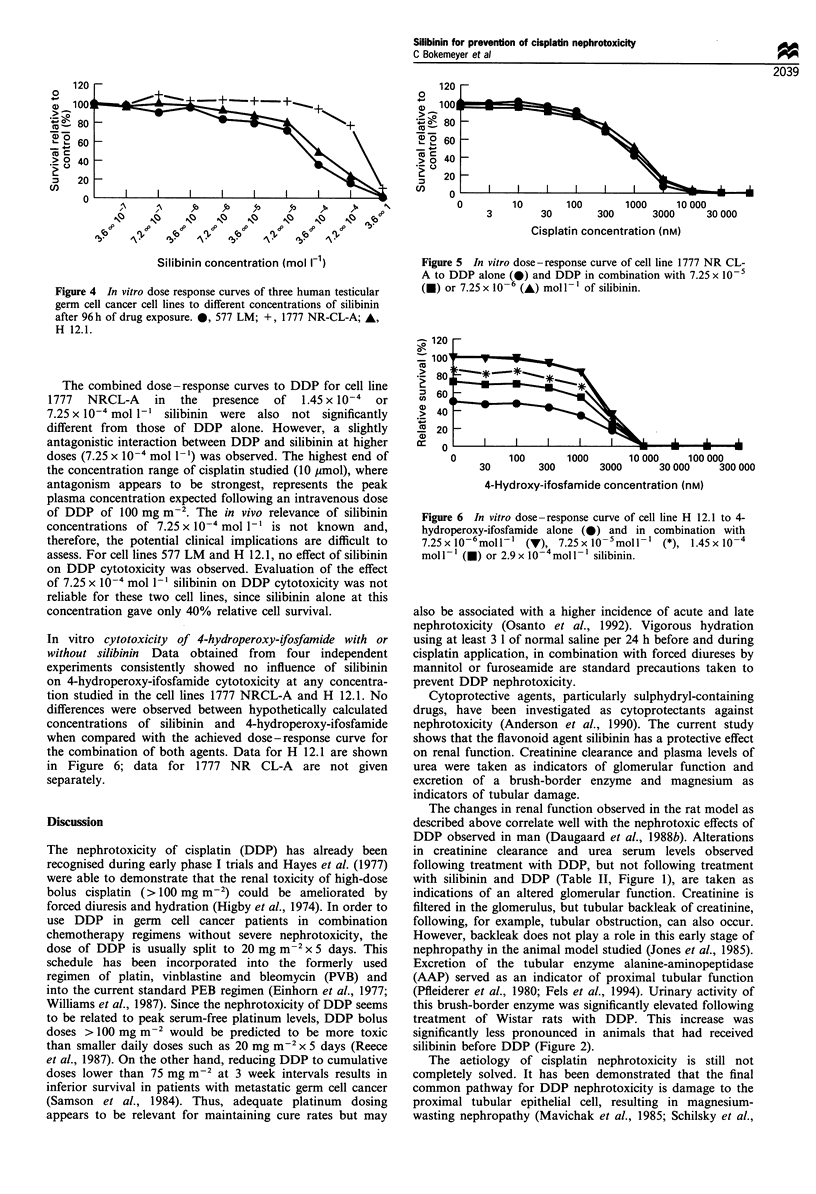
Abstract
Cisplatin is one of the most active cytotoxic agents in the treatment of testicular cancer, but its clinical use is associated with side-effects such as ototoxicity, neurotoxicity and nephrotoxicity. Long-term kidney damage from cisplatin particularly affects the proximal tubular apparatus and can be detected by increased urinary excretion of brush-border enzymes, such as L-alanine-aminopeptidase (AAP), and magnesium. In the current study, the flavonoid silibinin was used as a nephroprotectant for cisplatin-induced nephropathy in a rat animal model. Infusion of silibinin before cisplatin results in a significant decrease in glomerular (indicated by creatinine clearance and serum urea level) and tubular kidney toxicity (excretion of brush-border enzymes and magnesium). Silibinin given alone had no effect on renal function. In order to exclude an inhibition of the anti-tumour activity of cisplatin and 4-hydroperoxy-ifosfamide by co-administration of silibinin, in vitro studies were performed in three established human testicular cancer cell lines. Dose-response curves for cisplatin (3-30 000 nmol) combined with non-toxic silibinin doses (7.25 x 10(-6) or 7.25 x 10(-5) mol l-1) did not deviate significantly from those of cisplatin alone as measured by relative cell survival during a 5 day assay using the sulphorhodamine-B staining technique. Also silibinin did not influence the cytotoxic activity of 4-hydroperoxy-ifosfamide (30-10 000 nmol) in vitro. In summary, these in vitro data rule out a significant inhibition of the anti-tumour activity of the major nephrotoxic components, cisplatin and 4-hydroperoxy-ifosfamide, by co-administration of silibinin in a human germ cell tumour cell line model. Together with these demonstrated cytoprotection effects in the rat animal model, these data form the basis for a randomised clinical trial of silibinin for the protection of cisplatin-associated nephrotoxicity in patients with testicular cancer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aamdal S., Fodstad O., Pihl A. Some procedures to reduce cis-platinum toxicity reduce antitumour activity. Cancer Treat Rev. 1987 Dec;14(3-4):389–395. doi: 10.1016/0305-7372(87)90035-1. [DOI] [PubMed] [Google Scholar]
- Ammer U., Natochin Y. u., David C., Rumrich G., Ullrich K. J. Cisplatin nephrotoxicity: site of functional disturbance and correlation to loss of body weight. Ren Physiol Biochem. 1993 May-Jun;16(3):131–145. doi: 10.1159/000173759. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Naganuma A., Meister A. Protection against cisplatin toxicity by administration of glutathione ester. FASEB J. 1990 Nov;4(14):3251–3255. doi: 10.1096/fasebj.4.14.2227215. [DOI] [PubMed] [Google Scholar]
- Bajorin D. F., Sarosdy M. F., Pfister D. G., Mazumdar M., Motzer R. J., Scher H. I., Geller N. L., Fair W. R., Herr H., Sogani P. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993 Apr;11(4):598–606. doi: 10.1200/JCO.1993.11.4.598. [DOI] [PubMed] [Google Scholar]
- Bitran J. D., Desser R. K., Billings A. A., Kozloff M. F., Shapiro C. M. Acute nephrotoxicity following cis-dichlorodiammine-platinum. Cancer. 1982 May 1;49(9):1784–1788. doi: 10.1002/1097-0142(19820501)49:9<1784::aid-cncr2820490909>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C., Schmoll H. J., Ludwig E., Harstrick A., Dunn T., Casper J. The anti-tumour activity of ifosfamide on heterotransplanted testicular cancer cell lines remains unaltered by the uroprotector mesna. Br J Cancer. 1994 May;69(5):863–867. doi: 10.1038/bjc.1994.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bülles H., Bülles J., Krumbiegel G., Mennicke W. H., Nitz D. Untersuchungen zur Verstoffwechselung und zur Ausscheidung von Silybin bei der Ratte. Arzneimittelforschung. 1975 Jun;25(6):902–905. [PubMed] [Google Scholar]
- Casper J., Schmoll H. J., Schnaidt U., Fonatsch C. Cell lines of human germinal cancer. Int J Androl. 1987 Feb;10(1):105–113. doi: 10.1111/j.1365-2605.1987.tb00171.x. [DOI] [PubMed] [Google Scholar]
- Daugaard G., Abildgaard U., Holstein-Rathlou N. H., Bruunshuus I., Bucher D., Leyssac P. P. Renal tubular function in patients treated with high-dose cisplatin. Clin Pharmacol Ther. 1988 Aug;44(2):164–172. doi: 10.1038/clpt.1988.132. [DOI] [PubMed] [Google Scholar]
- Daugaard G., Holstein-Rathlou N. H., Leyssac P. P. Effect of cisplatin on proximal convoluted and straight segments of the rat kidney. J Pharmacol Exp Ther. 1988 Mar;244(3):1081–1085. [PubMed] [Google Scholar]
- Einhorn L. H., Donohue J. Cis-diamminedichloroplatinum, vinblastine, and bleomycin combination chemotherapy in disseminated testicular cancer. Ann Intern Med. 1977 Sep;87(3):293–298. doi: 10.7326/0003-4819-87-3-293. [DOI] [PubMed] [Google Scholar]
- Faulstich H., Jahn W., Wieland T. Silybin inhibition of amatoxin uptake in the perfused rat liver. Arzneimittelforschung. 1980;30(3):452–454. [PubMed] [Google Scholar]
- Ferenci P., Dragosics B., Dittrich H., Frank H., Benda L., Lochs H., Meryn S., Base W., Schneider B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989 Jul;9(1):105–113. doi: 10.1016/0168-8278(89)90083-4. [DOI] [PubMed] [Google Scholar]
- Field M. J., Bostrom T. E., Seow F., Györy A. Z., Cockayne D. J. Acute cisplatin nephrotoxicity in the rat. Evidence for impaired entry of sodium into proximal tubule cells. Pflugers Arch. 1989 Sep;414(6):647–650. doi: 10.1007/BF00582130. [DOI] [PubMed] [Google Scholar]
- Hacke M., Schmoll H. J., Alt J. M., Baumann K., Stolte H. Nephrotoxicity of cis-diamminedichloroplatinum with or without ifosfamide in cancer treatment. Clin Physiol Biochem. 1983;1(1):17–26. [PubMed] [Google Scholar]
- Hannemann J., Baumann K. Cisplatin-induced lipid peroxidation and decrease of gluconeogenesis in rat kidney cortex: different effects of antioxidants and radical scavengers. Toxicology. 1988 Oct;51(2-3):119–132. doi: 10.1016/0300-483x(88)90143-6. [DOI] [PubMed] [Google Scholar]
- Hansen S. W., Groth S., Daugaard G., Rossing N., Rørth M. Long-term effects on renal function and blood pressure of treatment with cisplatin, vinblastine, and bleomycin in patients with germ cell cancer. J Clin Oncol. 1988 Nov;6(11):1728–1731. doi: 10.1200/JCO.1988.6.11.1728. [DOI] [PubMed] [Google Scholar]
- Hayes D. M., Cvitkovic E., Golbey R. B., Scheiner E., Helson L., Krakoff I. H. High dose cis-platinum diammine dichloride: amelioration of renal toxicity by mannitol diuresis. Cancer. 1977 Apr;39(4):1372–1381. doi: 10.1002/1097-0142(197704)39:4<1372::aid-cncr2820390404>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Higby D. J., Wallace H. J., Jr, Albert D., Holland J. F. Diamminodichloroplatinum in the chemotherapy of testicular tumors. J Urol. 1974 Jul;112(1):100–104. doi: 10.1016/s0022-5347(17)59652-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Takayanagi Y., Sasaki K. Enhancement of cisplatin toxicity by buthionine sulfoximine, a glutathione-depleting agent, in mice. Res Commun Chem Pathol Pharmacol. 1990 Jan;67(1):131–141. [PubMed] [Google Scholar]
- Jones T. W., Chopra S., Kaufman J. S., Flamenbaum W., Trump B. F. Cis-diamminedichloroplatinum (II)-induced acute renal failure in the rat. Correlation of structural and functional alterations. Lab Invest. 1985 Apr;52(4):363–374. [PubMed] [Google Scholar]
- Lorenz D., Lücker P. W., Mennicke W. H., Wetzelsberger N. Pharmacokinetic studies with silymarin in human serum and bile. Methods Find Exp Clin Pharmacol. 1984 Oct;6(10):655–661. [PubMed] [Google Scholar]
- Mavichak V., Wong N. L., Quamme G. A., Magil A. B., Sutton R. A., Dirks J. H. Studies on the pathogenesis of cisplatin-induced hypomagnesemia in rats. Kidney Int. 1985 Dec;28(6):914–921. doi: 10.1038/ki.1985.217. [DOI] [PubMed] [Google Scholar]
- Middleton E., Jr, Kandaswami C. Effects of flavonoids on immune and inflammatory cell functions. Biochem Pharmacol. 1992 Mar 17;43(6):1167–1179. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- Mira L., Silva M., Manso C. F. Scavenging of reactive oxygen species by silibinin dihemisuccinate. Biochem Pharmacol. 1994 Aug 17;48(4):753–759. doi: 10.1016/0006-2952(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Munshi N. C., Loehrer P. J., Sr, Williams S. D., Langefeld C., Sledge G., Nichols C. R., Roth B. J., Neuman A., Walsh W. B., Einhorn L. H. Comparison of N-acetylcysteine and mesna as uroprotectors with ifosfamide combination chemotherapy in refractory germ cell tumors. Invest New Drugs. 1992 Aug;10(3):159–163. doi: 10.1007/BF00877240. [DOI] [PubMed] [Google Scholar]
- Osanto S., Bukman A., Van Hoek F., Sterk P. J., De Laat J. A., Hermans J. Long-term effects of chemotherapy in patients with testicular cancer. J Clin Oncol. 1992 Apr;10(4):574–579. doi: 10.1200/JCO.1992.10.4.574. [DOI] [PubMed] [Google Scholar]
- Pfleiderer G., Baier M., Mondorf A. W., Stefanescu T., Scherberich J. E., Müller H. Change in alkaline phosphatase isoenzyme pattern in urine as possible marker for renal disease. Kidney Int. 1980 Feb;17(2):242–249. doi: 10.1038/ki.1980.28. [DOI] [PubMed] [Google Scholar]
- Reece P. A., Stafford I., Russell J., Khan M., Gill P. G. Creatinine clearance as a predictor of ultrafilterable platinum disposition in cancer patients treated with cisplatin: relationship between peak ultrafilterable platinum plasma levels and nephrotoxicity. J Clin Oncol. 1987 Feb;5(2):304–309. doi: 10.1200/JCO.1987.5.2.304. [DOI] [PubMed] [Google Scholar]
- Sadzuka Y., Shoji T., Takino Y. Effect of cisplatin on the activities of enzymes which protect against lipid peroxidation. Biochem Pharmacol. 1992 Apr 15;43(8):1872–1875. doi: 10.1016/0006-2952(92)90725-x. [DOI] [PubMed] [Google Scholar]
- Safirstein R., Miller P., Dikman S., Lyman N., Shapiro C. Cisplatin nephrotoxicity in rats: defect in papillary hypertonicity. Am J Physiol. 1981 Aug;241(2):F175–F185. doi: 10.1152/ajprenal.1981.241.2.F175. [DOI] [PubMed] [Google Scholar]
- Samson M. K., Rivkin S. E., Jones S. E., Costanzi J. J., LoBuglio A. F., Stephens R. L., Gehan E. A., Cummings G. D. Dose-response and dose-survival advantage for high versus low-dose cisplatin combined with vinblastine and bleomycin in disseminated testicular cancer. A Southwest Oncology Group study. Cancer. 1984 Mar 1;53(5):1029–1035. doi: 10.1002/1097-0142(19840301)53:5<1029::aid-cncr2820530503>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Schilsky R. L., Barlock A., Ozols R. F. Persistent hypomagnesemia following cisplatin chemotherapy for testicular cancer. Cancer Treat Rep. 1982 Sep;66(9):1767–1769. [PubMed] [Google Scholar]
- Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990 Jul 4;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Sonnenbichler J., Zetl I. Biochemical effects of the flavonolignane silibinin on RNA, protein and DNA synthesis in rat livers. Prog Clin Biol Res. 1986;213:319–331. [PubMed] [Google Scholar]
- Tay L. K., Bregman C. L., Masters B. A., Williams P. D. Effects of cis-diamminedichloroplatinum(II) on rabbit kidney in vivo and on rabbit renal proximal tubule cells in culture. Cancer Res. 1988 May 1;48(9):2538–2543. [PubMed] [Google Scholar]
- Valenzuela A., Guerra R. Protective effect of the flavonoid silybin dihemisuccinate on the toxicity of phenylhydrazine on rat liver. FEBS Lett. 1985 Feb 25;181(2):291–294. doi: 10.1016/0014-5793(85)80278-7. [DOI] [PubMed] [Google Scholar]
- Valenzuela A., Lagos C., Schmidt K., Videla L. A. Silymarin protection against hepatic lipid peroxidation induced by acute ethanol intoxication in the rat. Biochem Pharmacol. 1985 Jun 15;34(12):2209–2212. doi: 10.1016/0006-2952(85)90421-6. [DOI] [PubMed] [Google Scholar]
- Wagner H., Diesel P., Seitz M. Zur Chemie und Analytik von Silymarin aus Siybum marianum Gaertn. Arzneimittelforschung. 1974 Apr;24(4):466–471. [PubMed] [Google Scholar]
- Williams S. D., Birch R., Einhorn L. H., Irwin L., Greco F. A., Loehrer P. J. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. 1987 Jun 4;316(23):1435–1440. doi: 10.1056/NEJM198706043162302. [DOI] [PubMed] [Google Scholar]


