Abstract
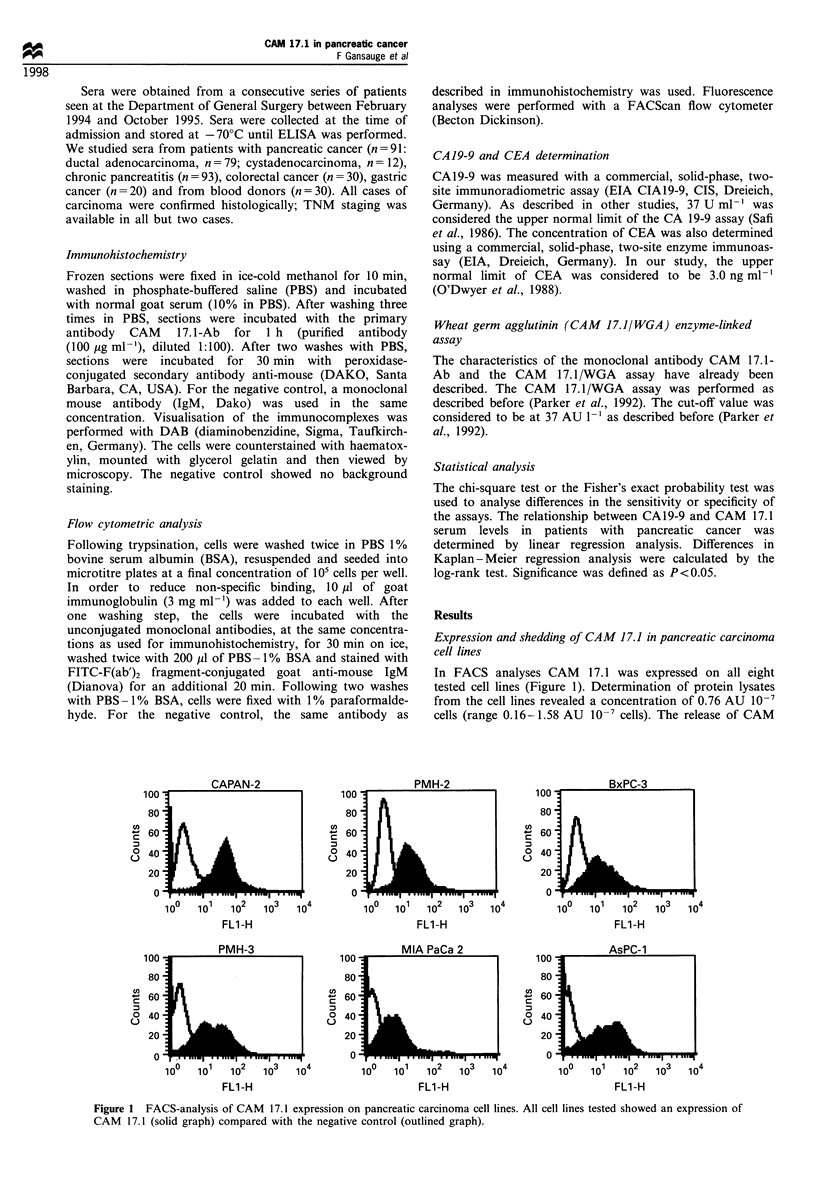
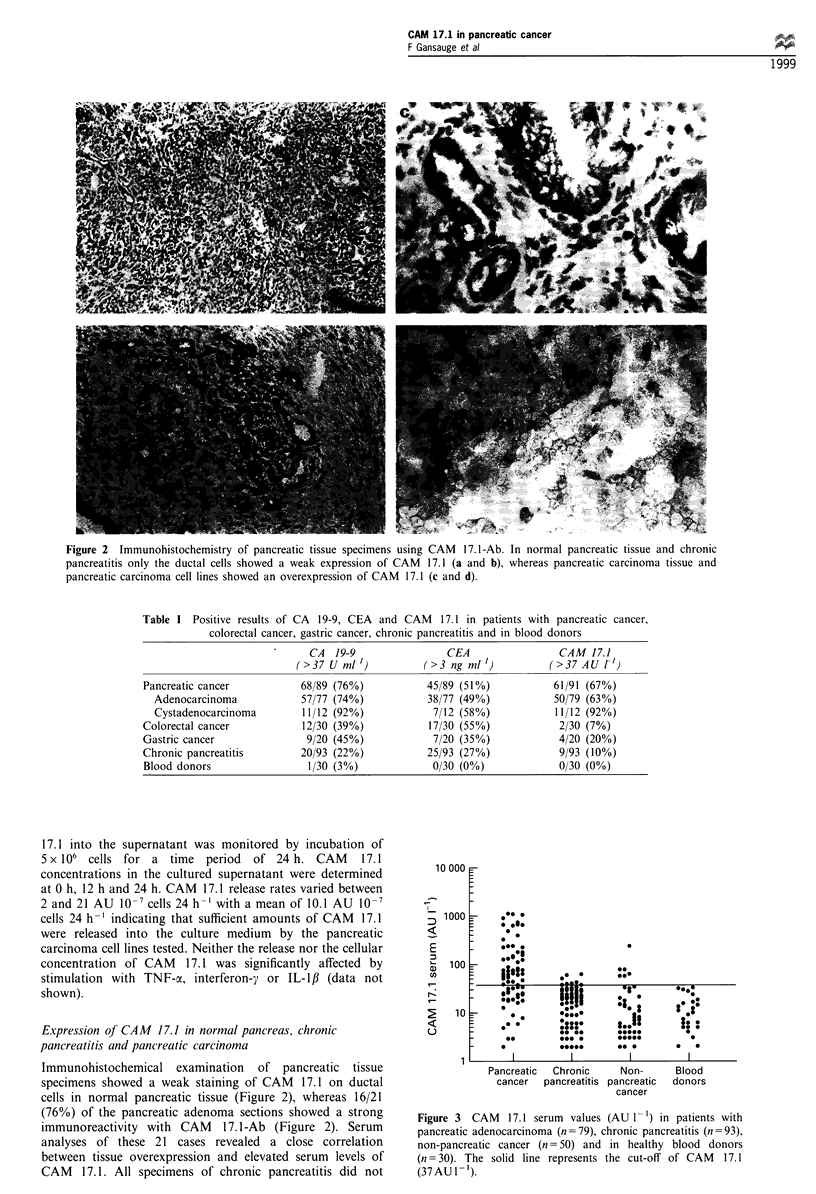
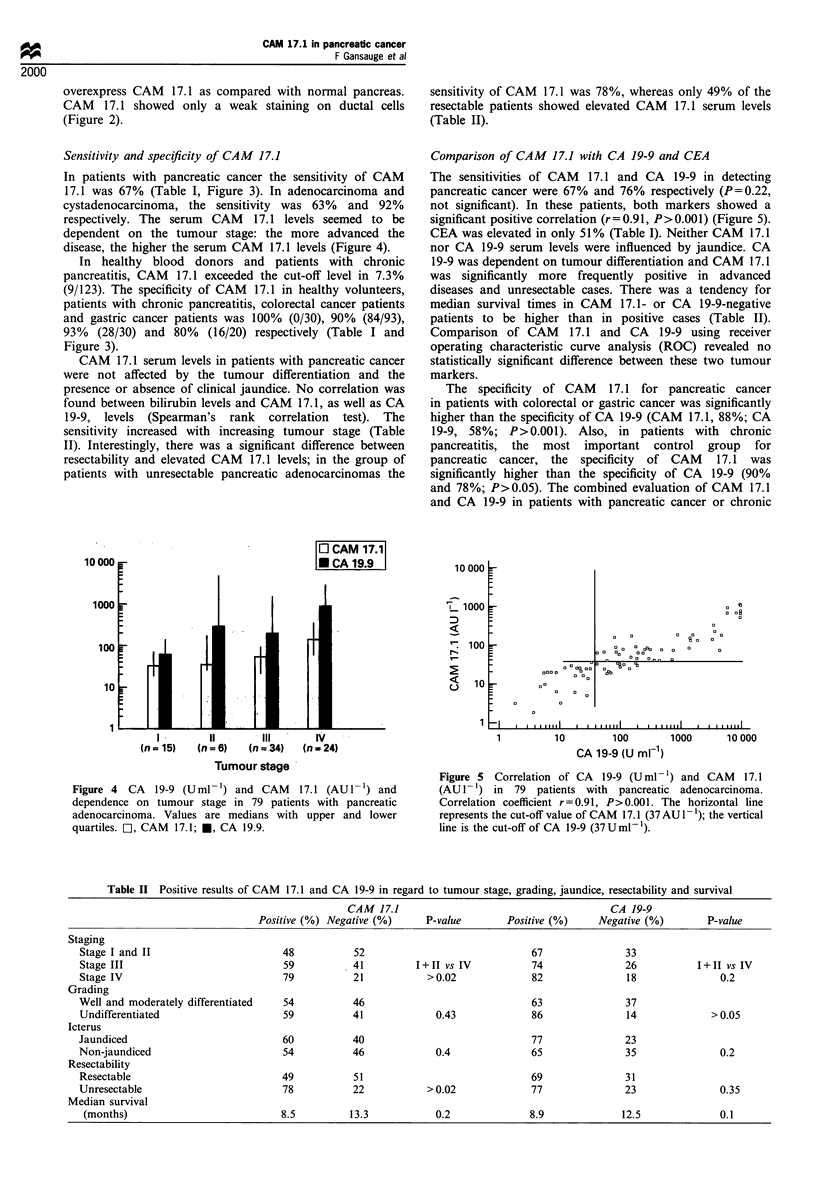
CAM 17.1-Ab is a recently described monoclonal antibody that detects a mucus glycoprotein with high specificity for intestinal mucus, particularly in the colon, small intestine, biliary tract and pancreas. We investigated the expression and release of CAM 17.1 in pancreatic carcinoma cell lines and tissue specimens of normal pancreas, chronic pancreatitis and pancreatic cancer. CAM 17.1 was weakly expressed on normal ductal cells and chronic pancreatitis, whereas it was overexpressed in pancreatic cancer. Serum analysis using a new enzyme-linked antibody sandwich assay (CAM 17.1/WGA) of patients with chronic pancreatitis, pancreatic cancer or other gastrointestinal cancer and of healthy blood donors revealed a high sensitivity (67%) and excellent specificity (90%) of CAM 17.1/WGA assay in pancreatic cancer. In comparison with the tumour marker CA19-9, the sensitivity of the CAM 17.1/WGA assay was similar to the sensitivity of CA 19-9 (67% and 76%, P = 0.22), whereas the specificity of CAM 17.1/WGA assay was higher than in CA 19-9 (90% compared with 78% in chronic pancreatitis, P > 0.05).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balagué C., Gambús G., Carrato C., Porchet N., Aubert J. P., Kim Y. S., Real F. X. Altered expression of MUC2, MUC4, and MUC5 mucin genes in pancreas tissues and cancer cell lines. Gastroenterology. 1994 Apr;106(4):1054–1061. doi: 10.1016/0016-5085(94)90767-6. [DOI] [PubMed] [Google Scholar]
- Ching C. K., Rhodes J. M. Identification and partial characterization of a new pancreatic cancer-related serum glycoprotein by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and lectin blotting. Gastroenterology. 1988 Jul;95(1):137–142. doi: 10.1016/0016-5085(88)90302-2. [DOI] [PubMed] [Google Scholar]
- Ching C. K., Rhodes J. M. Purification and characterization of a peanut-agglutinin-binding pancreatic-cancer-related serum mucus glycoprotein. Int J Cancer. 1990 Jun 15;45(6):1022–1027. doi: 10.1002/ijc.2910450607. [DOI] [PubMed] [Google Scholar]
- Frebourg T., Bercoff E., Manchon N., Senant J., Basuyau J. P., Breton P., Janvresse A., Brunelle P., Bourreille J. The evaluation of CA 19-9 antigen level in the early detection of pancreatic cancer. A prospective study of 866 patients. Cancer. 1988 Dec 1;62(11):2287–2290. doi: 10.1002/1097-0142(19881201)62:11<2287::aid-cncr2820621103>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Friess H., Büchler M., Auerbach B., Weber A., Malfertheiner P., Hammer K., Madry N., Greiner S., Bosslet K., Beger H. G. CA 494--a new tumor marker for the diagnosis of pancreatic cancer. Int J Cancer. 1993 Mar 12;53(5):759–763. doi: 10.1002/ijc.2910530509. [DOI] [PubMed] [Google Scholar]
- Gelder F. B., Reese C. J., Moossa A. R., Hall T., Hunter R. Purification, partial characterization, and clinical evaluation of a pancreatic oncofetal antigen. Cancer Res. 1978 Feb;38(2):313–324. [PubMed] [Google Scholar]
- Habib N. A., Hershman M. J., Haberland F., Papp L., Wood C. B., Williamson R. C. The use of CA-50 radioimmunoassay in differentiating benign and malignant pancreatic disease. Br J Cancer. 1986 May;53(5):697–699. doi: 10.1038/bjc.1986.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund C., Roberts P. J., Kuusela P., Scheinin T. M., Mäkelä O., Jalanko H. Evaluation of CA 19-9 as a serum tumour marker in pancreatic cancer. Br J Cancer. 1986 Feb;53(2):197–202. doi: 10.1038/bjc.1986.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliner M. A. Human nasal respiratory secretions and host defense. Am Rev Respir Dis. 1991 Sep;144(3 Pt 2):S52–S56. doi: 10.1164/ajrccm/144.3_pt_2.S52. [DOI] [PubMed] [Google Scholar]
- Kalser M. H., Barkin J. S., Redlhammer D., Heal A. Circulating carcinoembryonic antigen in pancreatic carcinoma. Cancer. 1978 Sep;42(3 Suppl):1468–1471. doi: 10.1002/1097-0142(197809)42:3+<1468::aid-cncr2820420816>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Gum J. R., Jr, Byrd J. C., Toribara N. W. The structure of human intestinal apomucins. Am Rev Respir Dis. 1991 Sep;144(3 Pt 2):S10–S14. doi: 10.1164/ajrccm/144.3_pt_2.S10. [DOI] [PubMed] [Google Scholar]
- Lucarotti M. E., Habib N. A., Kelly S. B., Rothnie N. D., Nelson O., Lindholm L., Cooper M. J., Wood C. B., Williamson R. C. Clinical evaluation of combined use of CEA, CA19-9 and CA50 in the serum of patients with pancreatic carcinoma. Eur J Surg Oncol. 1991 Feb;17(1):51–53. [PubMed] [Google Scholar]
- Magnani J. L., Steplewski Z., Koprowski H., Ginsburg V. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res. 1983 Nov;43(11):5489–5492. [PubMed] [Google Scholar]
- Makin C. A., Bobrow L. G., Bodmer W. F. Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol. 1984 Sep;37(9):975–983. doi: 10.1136/jcp.37.9.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O., Johansson C., Glimelius B., Persson B., Nørgaard-Pedersen B., Andrén-Sandberg A., Lindholm L. Sensitivity and specificity of CA242 in gastro-intestinal cancer. A comparison with CEA, CA50 and CA 19-9. Br J Cancer. 1992 Feb;65(2):215–221. doi: 10.1038/bjc.1992.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northover J. Carcinoembryonic antigen and recurrent colorectal cancer. Gut. 1986 Feb;27(2):117–122. doi: 10.1136/gut.27.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dwyer P. J., Mojzisik C., McCabe D. P., Farrar W. B., Carey L. C., Martin E. W., Jr Reoperation directed by carcinoembryonic antigen level: the importance of a thorough preoperative evaluation. Am J Surg. 1988 Feb;155(2):227–231. doi: 10.1016/s0002-9610(88)80699-8. [DOI] [PubMed] [Google Scholar]
- Ohshio G., Manabe T., Watanabe Y., Endo K., Kudo H., Suzuki T., Tobe T. Comparative studies of DU-PAN-2, carcinoembryonic antigen, and CA19-9 in the serum and bile of patients with pancreatic and biliary tract diseases: evaluation of the influence of obstructive jaundice. Am J Gastroenterol. 1990 Oct;85(10):1370–1376. [PubMed] [Google Scholar]
- Parker N., Makin C. A., Ching C. K., Eccleston D., Taylor O. M., Milton J. D., Rhodes J. M. A new enzyme-linked lectin/mucin antibody sandwich assay (CAM 17.1/WGA) assessed in combination with CA 19-9 and peanut lectin binding assay for the diagnosis of pancreatic cancer. Cancer. 1992 Sep 1;70(5):1062–1068. doi: 10.1002/1097-0142(19920901)70:5<1062::aid-cncr2820700509>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., McPhee M. S., Alpert E., Warshaw A. L., Isselbacher K. J. Galactosyltransferase isoenzyme II in the detection of pancreatic cancer: comparison with radiologic, endoscopic, and serologic tests. N Engl J Med. 1981 May 28;304(22):1313–1318. doi: 10.1056/NEJM198105283042201. [DOI] [PubMed] [Google Scholar]
- Raouf A., Parker N., Iddon D., Ryder S., Langdon-Brown B., Milton J. D., Walker R., Rhodes J. M. Ion exchange chromatography of purified colonic mucus glycoproteins in inflammatory bowel disease: absence of a selective subclass defect. Gut. 1991 Oct;32(10):1139–1145. doi: 10.1136/gut.32.10.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts P. F., Burns J. A histochemical study of mucins in normal and neoplastic human pancreatic tissue. J Pathol. 1972 Jun;107(2):87–94. doi: 10.1002/path.1711070203. [DOI] [PubMed] [Google Scholar]
- Russo A. J., Douglass H. O., Jr, Leveson S. H., Howell J. H., Holyoke E. D., Harvey S. R., Chu T. M., Goldrosen M. H. Evaluation of the microleukocyte adherence inhibition assay as an immunodiagnostic test for pancreatic cancer. Cancer Res. 1978 Jul;38(7):2023–2029. [PubMed] [Google Scholar]
- Safi F., Beger H. G., Bittner R., Büchler M., Krautzberger W. CA 19-9 and pancreatic adenocarcinoma. Cancer. 1986 Feb 15;57(4):779–783. doi: 10.1002/1097-0142(19860215)57:4<779::aid-cncr2820570417>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Schuessler M. H., Pintado S., Welt S., Real F. X., Xu M., Melamed M. R., Lloyd K. O., Oettgen H. F. Blood group and blood-group-related antigens in normal pancreas and pancreas cancer: enhanced expression of precursor type 1, Tn and sialyl-Tn in pancreas cancer. Int J Cancer. 1991 Jan 21;47(2):180–187. doi: 10.1002/ijc.2910470204. [DOI] [PubMed] [Google Scholar]
- Takahashi H. K., Metoki R., Hakomori S. Immunoglobulin G3 monoclonal antibody directed to Tn antigen (tumor-associated alpha-N-acetylgalactosaminyl epitope) that does not cross-react with blood group A antigen. Cancer Res. 1988 Aug 1;48(15):4361–4367. [PubMed] [Google Scholar]
- Toshkov I., Mogaki M., Kazakoff K., Pour P. M. The patterns of coexpression of tumor-associated antigens CA 19-9, TAG-72, and DU-PAN-2 in human pancreatic cancer. Int J Pancreatol. 1994 Apr;15(2):97–103. doi: 10.1007/BF02924659. [DOI] [PubMed] [Google Scholar]
- Warshaw A. L., Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992 Feb 13;326(7):455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- Warshaw A. L., Lee K. H., Wood W. C., Cohen A. M. Sensitivity and specificity of serum ribonuclease in the diagnosis of pancreatic cancer. Am J Surg. 1980 Jan;139(1):27–32. doi: 10.1016/0002-9610(80)90225-1. [DOI] [PubMed] [Google Scholar]
- Wesley A., Mantle M., Man D., Qureshi R., Forstner G., Forstner J. Neutral and acidic species of human intestinal mucin. Evidence for different core peptides. J Biol Chem. 1985 Jul 5;260(13):7955–7959. [PubMed] [Google Scholar]
- Xu M., Real F. X., Welt S., Schüssler M. H., Oettgen H. F., Old L. J. Expression of TAG-72 in normal colon, transitional mucosa, and colon cancer. Int J Cancer. 1989 Dec 15;44(6):985–989. doi: 10.1002/ijc.2910440607. [DOI] [PubMed] [Google Scholar]
- von Rosen A., Linder S., Harmenberg U., Pegert S. Serum levels of CA 19-9 and CA 50 in relation to Lewis blood cell status in patients with malignant and benign pancreatic disease. Pancreas. 1993 Mar;8(2):160–165. doi: 10.1097/00006676-199303000-00004. [DOI] [PubMed] [Google Scholar]



