Abstract
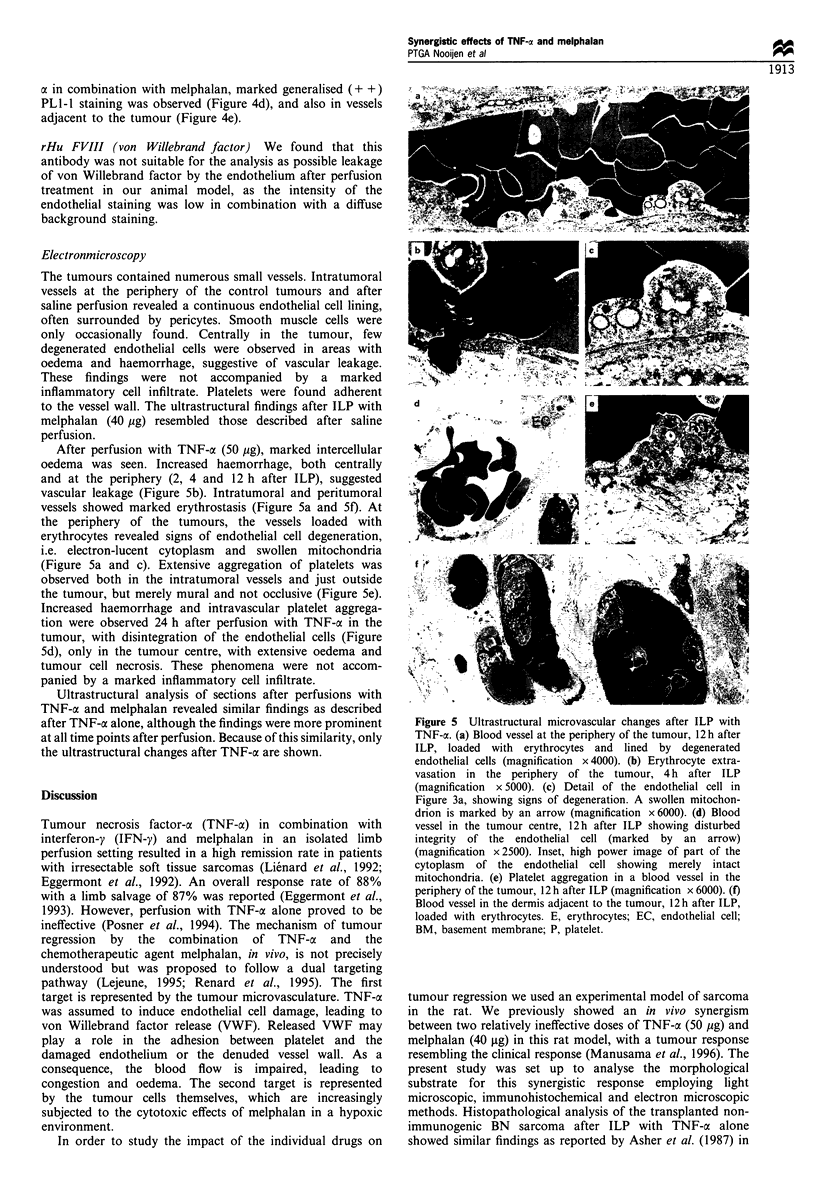
Isolated limb perfusion (ILP) with tumour necrosis factor alpha (TNF-alpha) and melphalan has shown impressive results in patients with irresectable soft tissue sarcomas and stage III melanoma of the extremities. The mechanisms of the reported in vivo synergistic anti-tumour effects of TNF-alpha and melphalan are not precisely understood. We have developed an ILP model in the rat using a non-immunogenic sarcoma in which similar in vivo synergy is observed. The aim of this present study was to analyse the morphological substrate for this synergistic response of TNF-alpha in combination with melphalan to shed more light on the pathomechanisms involved. Histology of the tumours from saline- (n = 14) and melphalan-treated (n = 11) rats revealed apparently vital tumour cells in over 80% of the cross-sections. Interstitial oedema and coagulation necrosis were observed in the remaining part of the tumour. Haemorrhage was virtually absent. TNF-alpha (n = 22) induced marked oedema, hyperaemia, vascular congestion, extravasation of erythrocytes and haemorrhagic necrosis (20-60% of the cross-sections). Oedema and haemorrhage suggested drastic alterations of permeability and integrity of the microvasculature. Using light and electron-microscopy, we observed that haemorrhage preceded generalised platelet aggregation. Therefore, we suggest that the observed platelet aggregation was the result of the microvascular damage rather than its initiator. Remarkably, these events hardly influenced tumour growth. However, perfusion with the combination of TNF-alpha and melphalan (n = 24) showed more extensive haemorrhagic necrosis (80-90% of the cross-sections) and revealed a prolonged remission (mean 11 days) in comparison with the other groups of rats. Electron microscopical analysis revealed similar findings as described after TNF-alpha alone, although the effects were more prominent at all time points after perfusion. In conclusion, our findings suggest that the enhanced anti-tumour effect after the combination of TNF-alpha with melphalan results from potentiation of the TNF-alpha-induced vascular changes accompanied by increased vascular permeability and platelet aggregation. This may result in additive cytotoxicity or inhibition of growth of residual tumour cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. B., Nelson W. G., Coffey D. S. Synergistic enhancement by tumor necrosis factor of in vitro cytotoxicity from chemotherapeutic drugs targeted at DNA topoisomerase II. Cancer Res. 1987 May 1;47(9):2403–2406. [PubMed] [Google Scholar]
- Asher A., Mulé J. J., Reichert C. M., Shiloni E., Rosenberg S. A. Studies on the anti-tumor efficacy of systemically administered recombinant tumor necrosis factor against several murine tumors in vivo. J Immunol. 1987 Feb 1;138(3):963–974. [PubMed] [Google Scholar]
- Bagchus W. M., Jeunink M. F., Rozing J., Elema J. D. A monoclonal antibody against rat platelets. I. Tissue distribution in vitro and in vivo. Clin Exp Immunol. 1989 Feb;75(2):317–323. [PMC free article] [PubMed] [Google Scholar]
- Benckhuysen C., Van Dijk W. J., Van't Hoff S. C. High-flow isolation perfusion of the rat hind limb in vivo. J Surg Oncol. 1982 Dec;21(4):249–257. doi: 10.1002/jso.2930210412. [DOI] [PubMed] [Google Scholar]
- Bertomeu M. C., Gallo S., Lauri D., Levine M. N., Orr F. W., Buchanan M. R. Chemotherapy enhances endothelial cell reactivity to platelets. Clin Exp Metastasis. 1990 Nov-Dec;8(6):511–518. doi: 10.1007/BF00135874. [DOI] [PubMed] [Google Scholar]
- Gerlach H., Lieberman H., Bach R., Godman G., Brett J., Stern D. Enhanced responsiveness of endothelium in the growing/motile state to tumor necrosis factor/cachectin. J Exp Med. 1989 Sep 1;170(3):913–931. doi: 10.1084/jem.170.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach H., Lieberman H., Bach R., Godman G., Brett J., Stern D. Enhanced responsiveness of endothelium in the growing/motile state to tumor necrosis factor/cachectin. J Exp Med. 1989 Sep 1;170(3):913–931. doi: 10.1084/jem.170.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Tacchini-Cottier F., Vesin C., Milon G., Lou J. N., Piguet P. F., Juillard P. TNF-induced microvascular pathology: active role for platelets and importance of the LFA-1/ICAM-1 interaction. Eur Cytokine Netw. 1993 Nov-Dec;4(6):415–419. [PubMed] [Google Scholar]
- Jain R. K. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6(4):559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987 Jun 15;47(12):3039–3051. [PubMed] [Google Scholar]
- Kachel D. L., Martin W. J., 2nd Cyclophosphamide-induced lung toxicity: mechanism of endothelial cell injury. J Pharmacol Exp Ther. 1994 Jan;268(1):42–46. [PubMed] [Google Scholar]
- Kuper C. F., Bloksma N., Bruyntjes J. P., Hofhuis F. M., Wolterink G. Effects of endotoxin-treatment on inflammatory cell infiltrates in murine Meth A sarcoma. J Pathol. 1985 Sep;147(1):41–48. doi: 10.1002/path.1711470106. [DOI] [PubMed] [Google Scholar]
- Lejeune F. J. High dose recombinant tumour necrosis factor (rTNF alpha) administered by isolation perfusion for advanced tumours of the limbs: a model for biochemotherapy of cancer. Eur J Cancer. 1995 Jun;31A(6):1009–1016. doi: 10.1016/0959-8049(94)00512-5. [DOI] [PubMed] [Google Scholar]
- Lienard D., Ewalenko P., Delmotte J. J., Renard N., Lejeune F. J. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992 Jan;10(1):52–60. doi: 10.1200/JCO.1992.10.1.52. [DOI] [PubMed] [Google Scholar]
- Liénard D., Eggermont A. M., Schraffordt Koops H., Kroon B. B., Rosenkaimer F., Autier P., Lejeune F. J. Isolated perfusion of the limb with high-dose tumour necrosis factor-alpha (TNF-alpha), interferon-gamma (IFN-gamma) and melphalan for melanoma stage III. Results of a multi-centre pilot study. Melanoma Res. 1994 Mar;4 (Suppl 1):21–26. [PubMed] [Google Scholar]
- MacPherson G. G., North R. J. Endotoxin-mediated necrosis and regression of established tumours in the mouse. A correlative study of quantitative changes in blood flow and ultrastructural morphology. Cancer Immunol Immunother. 1986;21(3):209–216. doi: 10.1007/BF00199364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manusama E. R., Nooijen P. T., Stavast J., Durante N. M., Marquet R. L., Eggermont A. M. Synergistic antitumour effect of recombinant human tumour necrosis factor alpha with melphalan in isolated limb perfusion in the rat. Br J Surg. 1996 Apr;83(4):551–555. doi: 10.1002/bjs.1800830438. [DOI] [PubMed] [Google Scholar]
- Marquet R. L., Schellekens H., Westbroek D. L., Jeekel J. Effect of treatment with interferon and cyclophosphamide on the growth of a spontaneous liposarcoma in rats. Int J Cancer. 1983 Feb 15;31(2):223–226. doi: 10.1002/ijc.2910310215. [DOI] [PubMed] [Google Scholar]
- Nooijen P. T., Eggermont A. M., Verbeek M. M., Schalkwijk L., Buurman W. A., de Waal R. M., Ruiter D. J. Transient induction of E-selectin expression following TNF alpha-based isolated limb perfusion in melanoma and sarcoma patients is not tumor specific. J Immunother Emphasis Tumor Immunol. 1996 Jan;19(1):33–44. doi: 10.1097/00002371-199601000-00004. [DOI] [PubMed] [Google Scholar]
- PREHN R. T., MAIN J. M. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957 Jun;18(6):769–778. [PubMed] [Google Scholar]
- Regenass U., Müller M., Curschellas E., Matter A. Anti-tumor effects of tumor necrosis factor in combination with chemotherapeutic agents. Int J Cancer. 1987 Feb 15;39(2):266–273. doi: 10.1002/ijc.2910390224. [DOI] [PubMed] [Google Scholar]
- Renard N., Liénard D., Lespagnard L., Eggermont A., Heimann R., Lejeune F. Early endothelium activation and polymorphonuclear cell invasion precede specific necrosis of human melanoma and sarcoma treated by intravascular high-dose tumour necrosis factor alpha (rTNF alpha). Int J Cancer. 1994 Jun 1;57(5):656–663. doi: 10.1002/ijc.2910570508. [DOI] [PubMed] [Google Scholar]
- Renard N., Nooijen P. T., Schalkwijk L., De Waal R. M., Eggermont A. M., Liénard D., Kroon B. B., Lejeune F. J., Ruiter D. J. VWF release and platelet aggregation in human melanoma after perfusion with TNF alpha. J Pathol. 1995 Jul;176(3):279–287. doi: 10.1002/path.1711760310. [DOI] [PubMed] [Google Scholar]
- Sato N., Goto T., Haranaka K., Satomi N., Nariuchi H., Mano-Hirano Y., Sawasaki Y. Actions of tumor necrosis factor on cultured vascular endothelial cells: morphologic modulation, growth inhibition, and cytotoxicity. J Natl Cancer Inst. 1986 Jun;76(6):1113–1121. [PubMed] [Google Scholar]
- Shimomura K., Manda T., Mukumoto S., Kobayashi K., Nakano K., Mori J. Recombinant human tumor necrosis factor-alpha: thrombus formation is a cause of anti-tumor activity. Int J Cancer. 1988 Feb 15;41(2):243–247. doi: 10.1002/ijc.2910410215. [DOI] [PubMed] [Google Scholar]
- Van de Wiel P. A., Bloksma N., Kuper C. F., Hofhuis F. M., Willers J. M. Macroscopic and microscopic early effects of tumour necrosis factor on murine Meth A sarcoma, and relation to curative activity. J Pathol. 1989 Jan;157(1):65–73. doi: 10.1002/path.1711570109. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Niitsu Y., Umeno H., Kuriyama H., Neda H., Yamauchi N., Maeda M., Urushizaki I. Toxic effect of tumor necrosis factor on tumor vasculature in mice. Cancer Res. 1988 Apr 15;48(8):2179–2183. [PubMed] [Google Scholar]
- de Kossodo S., Moore R., Gschmeissner S., East N., Upton C., Balkwill F. R. Changes in endogenous cytokines, adhesion molecules and platelets during cytokine-induced tumour necrosis. Br J Cancer. 1995 Nov;72(5):1165–1172. doi: 10.1038/bjc.1995.481. [DOI] [PMC free article] [PubMed] [Google Scholar]