Abstract
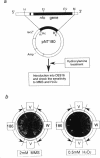
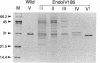
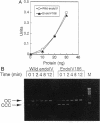
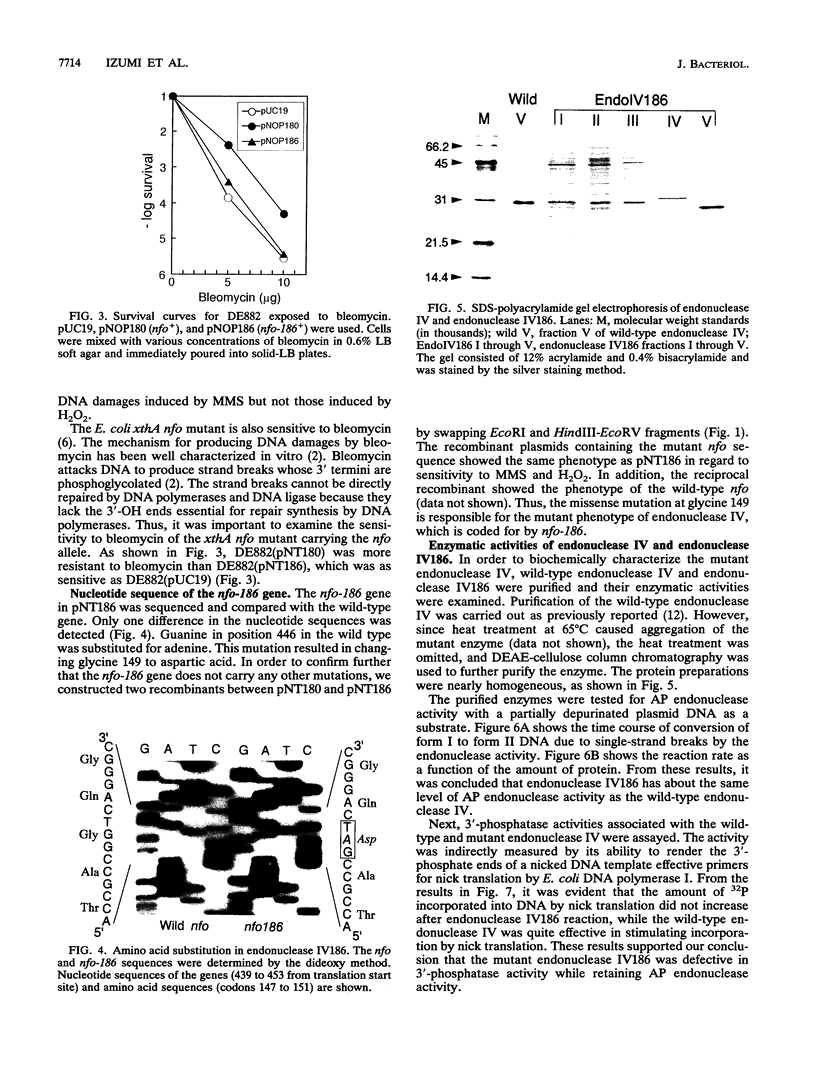
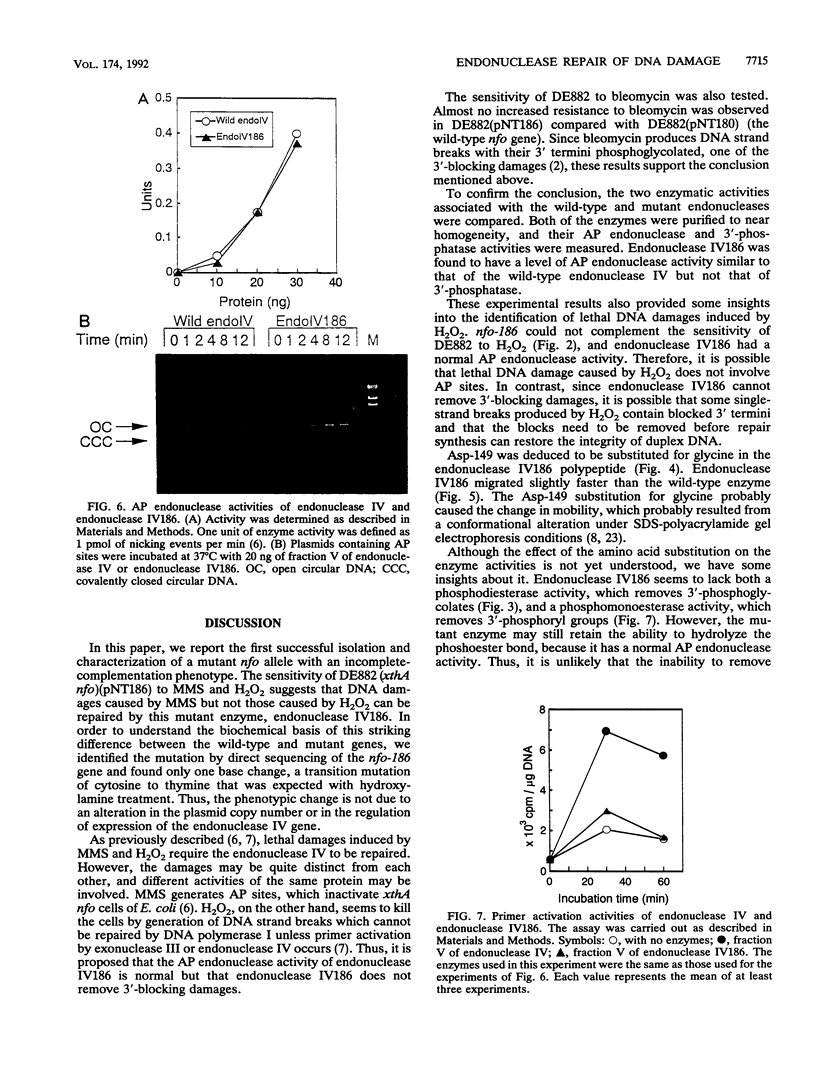
A mutant allele of the Escherichia coli nfo gene encoding endonuclease IV, nfo-186, was cloned into plasmid pUC18. When introduced into an E. coli xthA nfo mutant, the gene product of nfo-186 complemented the hypersensitivity of the mutant to methyl methanesulfonate (MMS) but not to hydrogen peroxide (H2O2) and bleomycin. These results suggest that the mutant endonuclease IV has normal activity for repairing DNA damages induced by MMS but not those induced by H2O2 and bleomycin. A missense mutation in the cloned nfo-186 gene, in which the wild-type glycine 149 was replaced by aspartic acid, was detected by DNA sequencing. The wild-type and mutant endonuclease IV were purified to near homogeneity, and their apurinic (AP) endonuclease and 3'-phosphatase activities were determined. No difference was observed in the AP endonuclease activities of the wild-type and mutant proteins. However, 3'-phosphatase activity was dramatically reduced in the mutant protein. From these results, it is concluded that the endonuclease IV186 protein is specifically deficient in the ability to remove 3'-terminus-blocking damage, which is required for DNA repair synthesis, and it is possible that the lethal DNA damage by H2O2 is 3'-blocking damage and not AP-site damage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruoma O. I., Halliwell B., Gajewski E., Dizdaroglu M. Damage to the bases in DNA induced by hydrogen peroxide and ferric ion chelates. J Biol Chem. 1989 Dec 5;264(34):20509–20512. [PubMed] [Google Scholar]
- Burger R. M., Projan S. J., Horwitz S. B., Peisach J. The DNA cleavage mechanism of iron-bleomycin. Kinetic resolution of strand scission from base propenal release. J Biol Chem. 1986 Dec 5;261(34):15955–15959. [PubMed] [Google Scholar]
- Busby S., Aiba H., de Crombrugghe B. Mutations in the Escherichia coli operon that define two promoters and the binding site of the cyclic AMP receptor protein. J Mol Biol. 1982 Jan 15;154(2):211–227. doi: 10.1016/0022-2836(82)90061-4. [DOI] [PubMed] [Google Scholar]
- Chan E., Weiss B. Endonuclease IV of Escherichia coli is induced by paraquat. Proc Natl Acad Sci U S A. 1987 May;84(10):3189–3193. doi: 10.1073/pnas.84.10.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Cunningham R. P., Saporito S. M., Spitzer S. G., Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Johnson A., Fung D. Exonuclease III and endonuclease IV remove 3' blocks from DNA synthesis primers in H2O2-damaged Escherichia coli. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7731–7735. doi: 10.1073/pnas.83.20.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano O., Aldrich T., Tamanoi F., Taparowsky E., Furth M., Wigler M. Analysis of the transforming potential of the human H-ras gene by random mutagenesis. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4008–4012. doi: 10.1073/pnas.81.13.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Kasai H., Crain P. F., Kuchino Y., Nishimura S., Ootsuyama A., Tanooka H. Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis. 1986 Nov;7(11):1849–1851. doi: 10.1093/carcin/7.11.1849. [DOI] [PubMed] [Google Scholar]
- Kuwabara M., Hiraoka W., Sato F. Evidence for the formation of strand-break precursors in hydroxy-attacked thymidine 5'-monophosphate by the spin trapping method. Biochemistry. 1989 Dec 12;28(25):9625–9632. doi: 10.1021/bi00451a013. [DOI] [PubMed] [Google Scholar]
- Levin J. D., Johnson A. W., Demple B. Homogeneous Escherichia coli endonuclease IV. Characterization of an enzyme that recognizes oxidative damage in DNA. J Biol Chem. 1988 Jun 15;263(17):8066–8071. [PubMed] [Google Scholar]
- Ljungquist S. A new endonuclease from Escherichia coli acting at apurinic sites in DNA. J Biol Chem. 1977 May 10;252(9):2808–2814. [PubMed] [Google Scholar]
- Ljungquist S., Lindahl T., Howard-Flanders P. Methyl methane sulfonate-sensitive mutant of Escherichia coli deficient in an endonuclease specific for apurinic sites in deoxyribonucleic acid. J Bacteriol. 1976 May;126(2):646–653. doi: 10.1128/jb.126.2.646-653.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen P. C., Triggs B. L., George C. S., Hrabarchuk B. E. Genetic mapping of katG, a locus that affects synthesis of the bifunctional catalase-peroxidase hydroperoxidase I in Escherichia coli. J Bacteriol. 1985 May;162(2):661–667. doi: 10.1128/jb.162.2.661-667.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzel-Landbeck L., Schutz G., Hagen U. In vitro repair of radiation-induced strand breaks in DNA. Biochim Biophys Acta. 1976 May 3;432(2):145–153. doi: 10.1016/0005-2787(76)90156-8. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., LEHMAN I. R., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. II. CHARACTERIZATION OF THE EXONUCLEASE ACTIVITY. J Biol Chem. 1964 Jan;239:251–258. [PubMed] [Google Scholar]
- Rogers S. G., Weiss B. Exonuclease III of Escherichia coli K-12, an AP endonuclease. Methods Enzymol. 1980;65(1):201–211. doi: 10.1016/s0076-6879(80)65028-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito S. M., Cunningham R. P. Nucleotide sequence of the nfo gene of Escherichia coli K-12. J Bacteriol. 1988 Nov;170(11):5141–5145. doi: 10.1128/jb.170.11.5141-5145.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito S. M., Gedenk M., Cunningham R. P. Role of exonuclease III and endonuclease IV in repair of pyrimidine dimers initiated by bacteriophage T4 pyrimidine dimer-DNA glycosylase. J Bacteriol. 1989 May;171(5):2542–2546. doi: 10.1128/jb.171.5.2542-2546.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H., Colby W. W., Capon D. J., Goeddel D. V., Levinson A. D. Biological properties of human c-Ha-ras1 genes mutated at codon 12. Nature. 1984 Nov 1;312(5989):71–75. doi: 10.1038/312071a0. [DOI] [PubMed] [Google Scholar]
- Siwek B., Bricteux-Grégoire S., Bailly V., Verly W. G. The relative importance of Escherichia coli exonuclease III and endonuclease IV for the hydrolysis of 3'-phosphoglycolate ends in polydeoxynucleotides. Nucleic Acids Res. 1988 Jun 10;16(11):5031–5038. doi: 10.1093/nar/16.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Endonuclease II of Escherichia coli is exonuclease III. J Biol Chem. 1976 Apr 10;251(7):1896–1901. [PubMed] [Google Scholar]
- White B. J., Hochhauser S. J., Cintron N. M., Weiss B. Genetic mapping of xthA, the structural gene for exonuclease III in Escherichia coli K-12. J Bacteriol. 1976 Jun;126(3):1082–1088. doi: 10.1128/jb.126.3.1082-1088.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]