Abstract
Etoposide (VP-16) is an anti-cancer drug commonly used against several types of tumours and leukaemia, either alone or in combination chemotherapy. Photodynamic therapy (PDT) is another, relatively new modality for treatment of various malignancies. The interactions between VP-16 and PDT, using aluminium tetrasulphophthalocyanine as photosensitiser, in K562 human leukaemic cells were investigated. Cell responses to individual and combined drug treatment under different experimental conditions revealed synergistic drug toxicity. The latter was evident from various events of cell response, including supra-additive accumulation of cells in G2/M cell cycle phase and endonucleolytic DNA fragmentation (apoptosis). The involvement of the cellular antioxidant system in the synergistic interactions of photosensitisation and VP-16 is proposed.
Full text
PDF

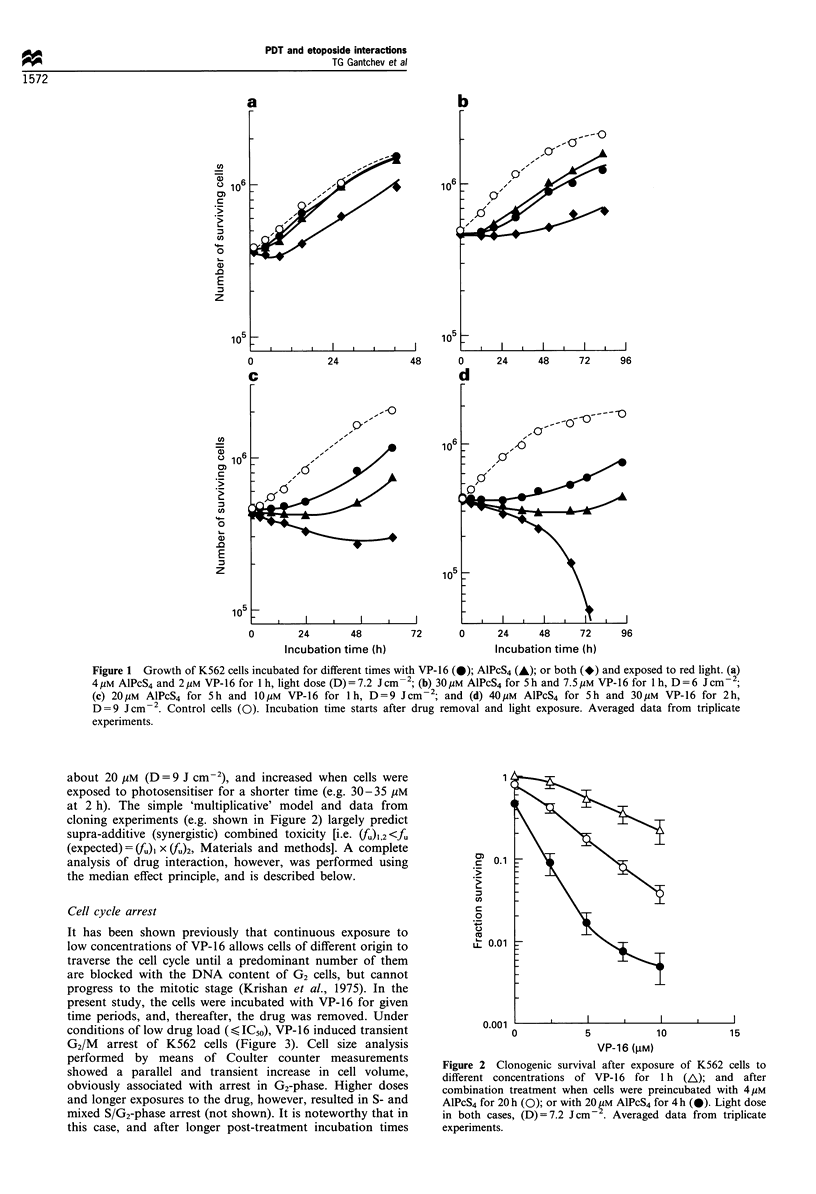
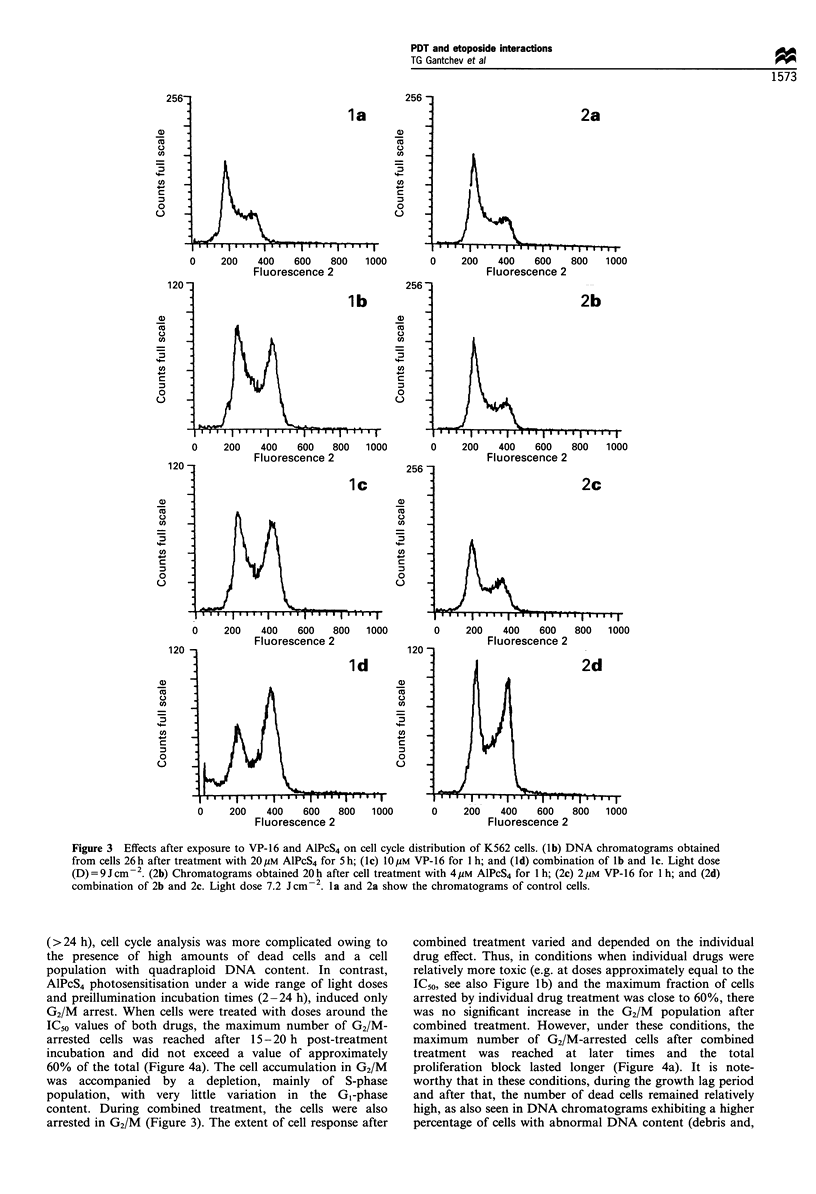
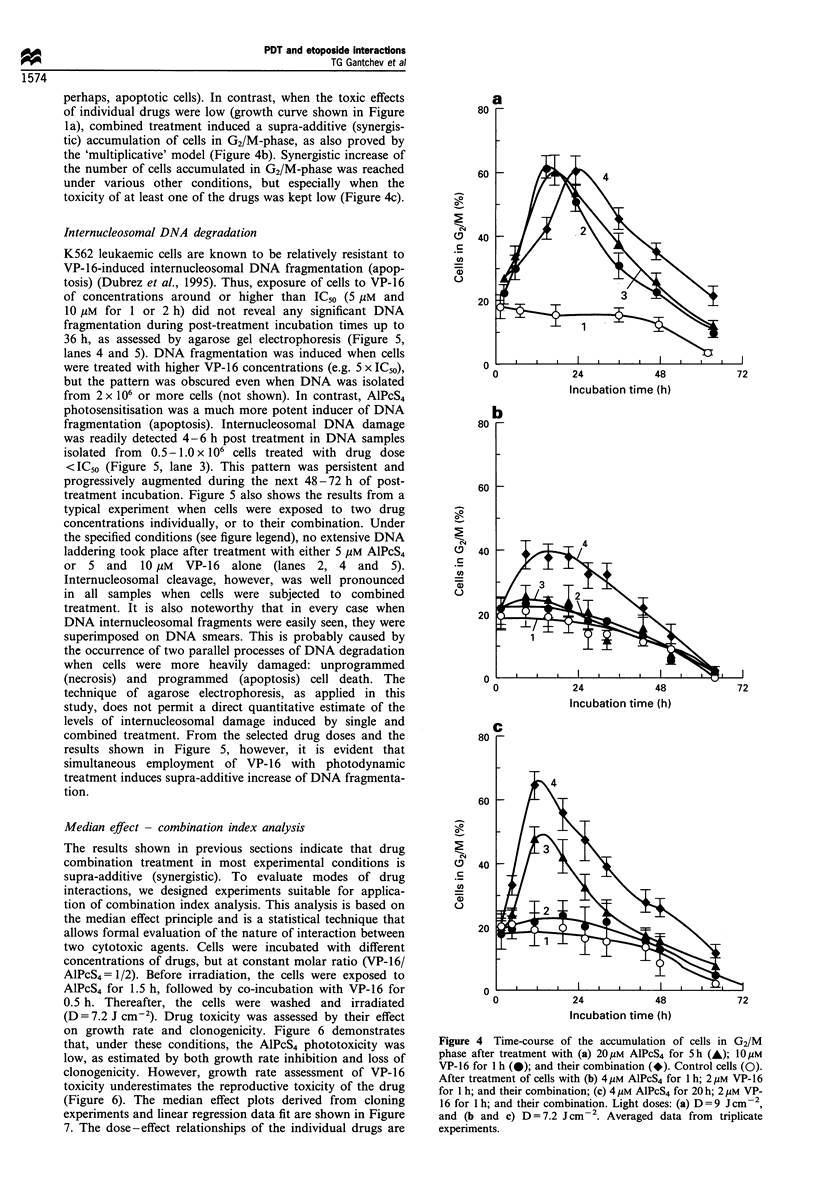
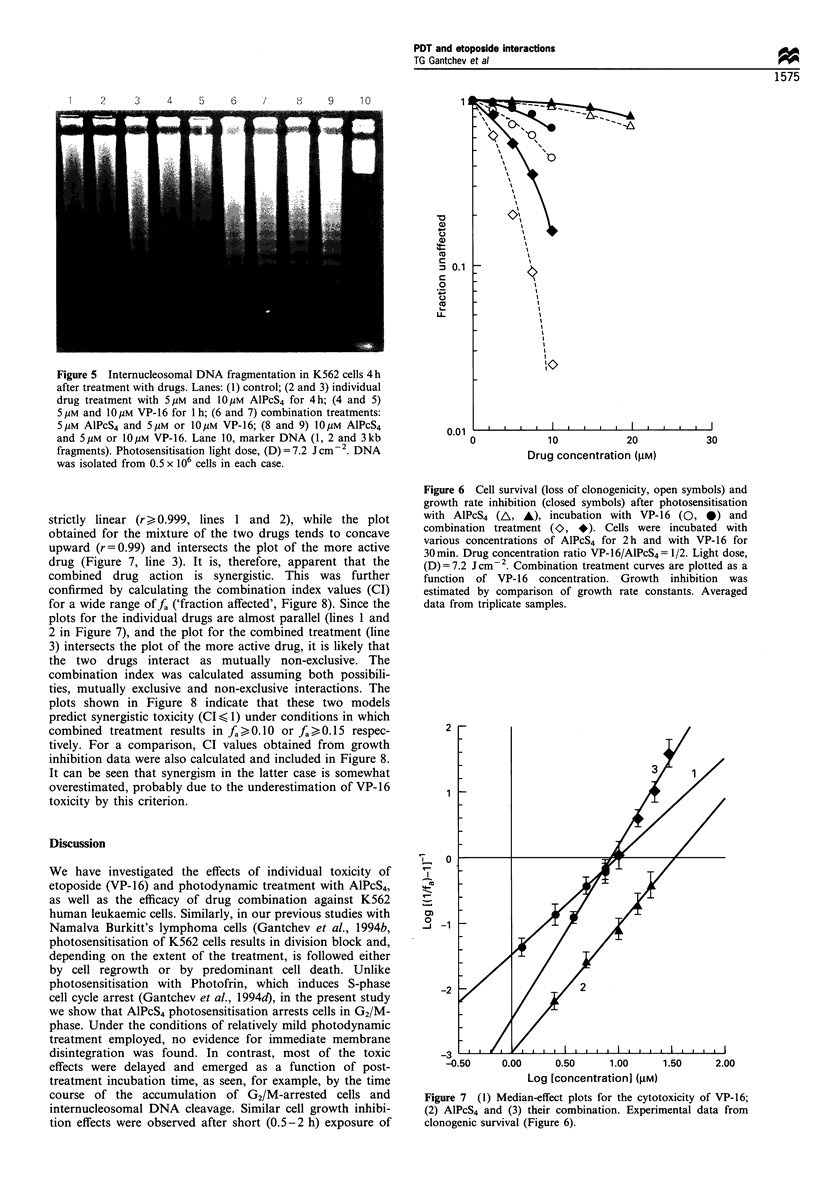
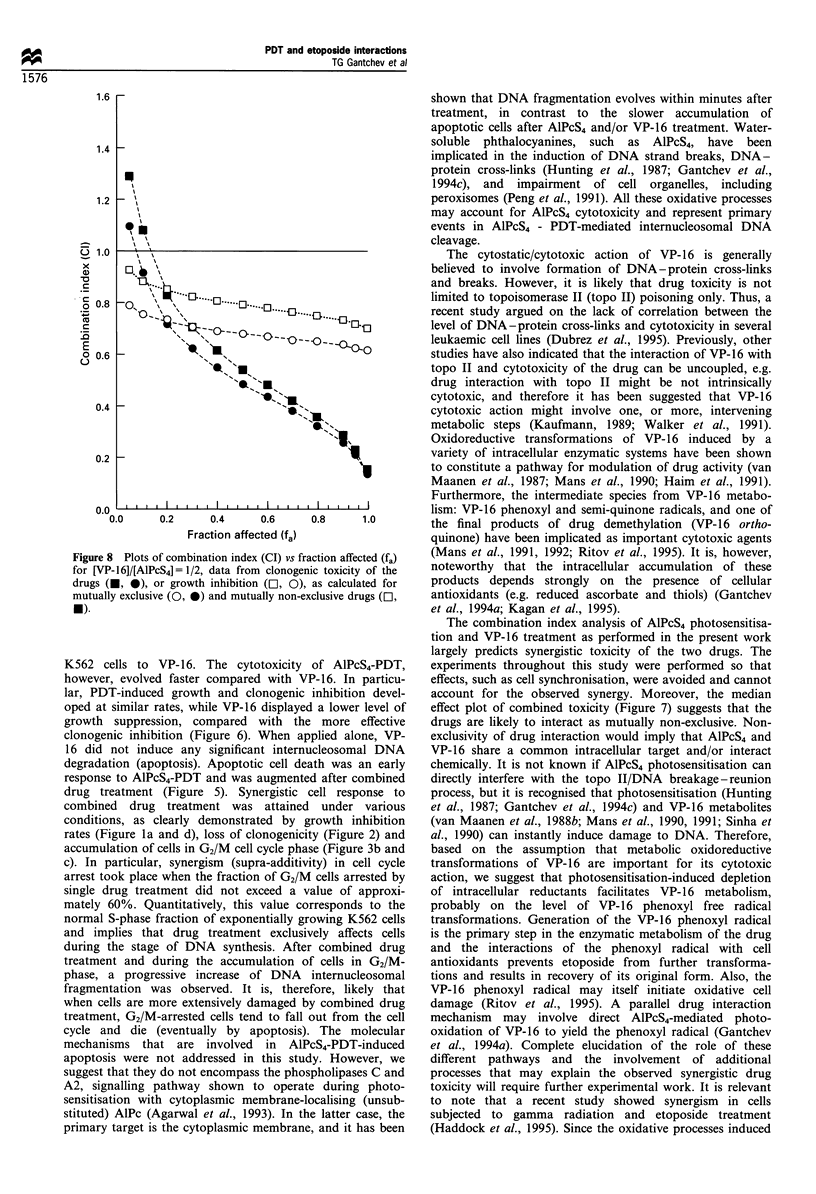

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal M. L., Larkin H. E., Zaidi S. I., Mukhtar H., Oleinick N. L. Phospholipase activation triggers apoptosis in photosensitized mouse lymphoma cells. Cancer Res. 1993 Dec 15;53(24):5897–5902. [PubMed] [Google Scholar]
- Aisner J., Lee E. J. Etoposide. Current and future status. Cancer. 1991 Jan 1;67(1 Suppl):215–219. doi: 10.1002/1097-0142(19910101)67:1+<215::aid-cncr2820671302>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Buettner G. R. Thiyl free radical production with hematoporphyrin derivative, cysteine and light: a spin-trapping study. FEBS Lett. 1984 Nov 19;177(2):295–299. doi: 10.1016/0014-5793(84)81303-4. [DOI] [PubMed] [Google Scholar]
- Champlin R., Gale R. P. Acute myelogenous leukemia: recent advances in therapy. Blood. 1987 Jun;69(6):1551–1562. [PubMed] [Google Scholar]
- Chou T. C., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Dubrez L., Goldwasser F., Genne P., Pommier Y., Solary E. The role of cell cycle regulation and apoptosis triggering in determining the sensitivity of leukemic cells to topoisomerase I and II inhibitors. Leukemia. 1995 Jun;9(6):1013–1024. [PubMed] [Google Scholar]
- Gantchev T. G., Gowans B. J., Hunting D. J., Wagner J. R., van Lier J. E. DNA strand scission and base release photosensitized by metallo-phthalocyanines. Int J Radiat Biol. 1994 Dec;66(6):705–716. [PubMed] [Google Scholar]
- Gantchev T. G., Urumov I. J., Hunting D. J., Van Lier J. E. Photocytotoxicity and intracellular generation of free radicals by tetrasulphonated Al- and Zn-phthalocyanines. Int J Radiat Biol. 1994 Mar;65(3):289–298. doi: 10.1080/09553009414550341. [DOI] [PubMed] [Google Scholar]
- Gantchev T. G., Urumov I. J., Van Lier J. E. On the relationship between rate of uptake of Photofrin and cellular responses to photodynamic treatment in vitro. Cancer Biochem Biophys. 1994 Apr;14(1):23–34. [PubMed] [Google Scholar]
- Gantchev T. G., van Lier J. E. Catalase inactivation following photosensitization with tetrasulfonated metallophthalocyanines. Photochem Photobiol. 1995 Jul;62(1):123–134. doi: 10.1111/j.1751-1097.1995.tb05248.x. [DOI] [PubMed] [Google Scholar]
- Gantchev T. G., van Lier J. E., Stoyanovsky D. A., Yalowich J. C., Kagan V. E. Interactions of phenoxyl radical of antitumor drug, etoposide, with reductants in solution and in cell and nuclear homogenates: electron spin resonance and high-performance liquid chromatography. Methods Enzymol. 1994;234:631–642. doi: 10.1016/0076-6879(94)34134-6. [DOI] [PubMed] [Google Scholar]
- Glisson B. S., Ross W. E. DNA topoisomerase II: a primer on the enzyme and its unique role as a multidrug target in cancer chemotherapy. Pharmacol Ther. 1987;32(2):89–106. doi: 10.1016/0163-7258(87)90054-4. [DOI] [PubMed] [Google Scholar]
- Haddock M. G., Ames M. M., Bonner J. A. Assessing the interaction of irradiation with etoposide or idarubicin. Mayo Clin Proc. 1995 Nov;70(11):1053–1060. doi: 10.4065/70.11.1053. [DOI] [PubMed] [Google Scholar]
- Haim N., Nemec J., Roman J., Sinha B. K. Peroxidase-catalyzed metabolism of etoposide (VP-16-213) and covalent binding of reactive intermediates to cellular macromolecules. Cancer Res. 1987 Nov 15;47(22):5835–5840. [PubMed] [Google Scholar]
- Hunting D. J., Gowans B. J., Brasseur N., van Lier J. E. DNA damage and repair following treatment of V-79 cells with sulfonated phthalocyanines. Photochem Photobiol. 1987 Jun;45(6):769–773. doi: 10.1111/j.1751-1097.1987.tb07880.x. [DOI] [PubMed] [Google Scholar]
- Kagan V. E., Yalowich J. C., Day B. W., Goldman R., Gantchev T. G., Stoyanovsky D. A. Ascorbate is the primary reductant of the phenoxyl radical of etoposide in the presence of thiols both in cell homogenates and in model systems. Biochemistry. 1994 Aug 16;33(32):9651–9660. doi: 10.1021/bi00198a034. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989 Nov 1;49(21):5870–5878. [PubMed] [Google Scholar]
- Krishan A., Paika K., Frei E., 3rd Cytofluorometric studies on the action of podophyllotoxin and epipodophyllotoxins (VM-26, VP-16-213) on the cell cycle traverse of human lymphoblasts. J Cell Biol. 1975 Sep;66(3):521–530. doi: 10.1083/jcb.66.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Mans D. R., Lafleur M. V., Westmijze E. J., Horn I. R., Bets D., Schuurhuis G. J., Lankelma J., Retèl J. Reactions of glutathione with the catechol, the ortho-quinone and the semi-quinone free radical of etoposide. Consequences for DNA inactivation. Biochem Pharmacol. 1992 Apr 15;43(8):1761–1768. doi: 10.1016/0006-2952(92)90708-q. [DOI] [PubMed] [Google Scholar]
- Mans D. R., Lafleur M. V., Westmijze E. J., van Maanen J. M., van Schaik M. A., Lankelma J., Retèl J. Formation of different reaction products with single- and double-stranded DNA by the ortho-quinone and the semi-quinone free radical of etoposide (VP-16-213). Biochem Pharmacol. 1991 Nov 6;42(11):2131–2139. doi: 10.1016/0006-2952(91)90348-9. [DOI] [PubMed] [Google Scholar]
- Mans D. R., Retèl J., van Maanen J. M., Lafleur M. V., van Schaik M. A., Pinedo H. M., Lankelma J. Role of the semi-quinone free radical of the anti-tumour agent etoposide (VP-16-213) in the inactivation of single- and double-stranded phi X174 DNA. Br J Cancer. 1990 Jul;62(1):54–60. doi: 10.1038/bjc.1990.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q., Farrants G. W., Madslien K., Bommer J. C., Moan J., Danielsen H. E., Nesland J. M. Subcellular localization, redistribution and photobleaching of sulfonated aluminum phthalocyanines in a human melanoma cell line. Int J Cancer. 1991 Sep 9;49(2):290–295. doi: 10.1002/ijc.2910490225. [DOI] [PubMed] [Google Scholar]
- Ritov V. B., Goldman R., Stoyanovsky D. A., Menshikova E. V., Kagan V. E. Antioxidant paradoxes of phenolic compounds: peroxyl radical scavenger and lipid antioxidant, etoposide (VP-16), inhibits sarcoplasmic reticulum Ca(2+)-ATPase via thiol oxidation by its phenoxyl radical. Arch Biochem Biophys. 1995 Aug 1;321(1):140–152. doi: 10.1006/abbi.1995.1379. [DOI] [PubMed] [Google Scholar]
- Shopova M., Gantchev T. Comparison of the photosensitizing efficiencies of haematoporphyrin (HP) and its derivative (HPD) with that of free-base tetrasulphophthalocyanine (TSPC-H2) in homogeneous and microheterogeneous media. J Photochem Photobiol B. 1990 Jun;6(1-2):49–59. doi: 10.1016/1011-1344(90)85073-6. [DOI] [PubMed] [Google Scholar]
- Sinha B. K., Antholine W. M., Kalyanaraman B., Eliot H. M. Copper ion-dependent oxy-radical mediated DNA damage from dihydroxy derivative of etoposide. Biochim Biophys Acta. 1990 Nov 14;1096(1):81–83. doi: 10.1016/0925-4439(90)90015-h. [DOI] [PubMed] [Google Scholar]
- Valeriote F., Lin H. s. Synergistic interaction of anticancer agents: a cellular perspective. Cancer Chemother Rep. 1975 Sep-Oct;59(5):895–900. [PubMed] [Google Scholar]
- Walker P. R., Smith C., Youdale T., Leblanc J., Whitfield J. F., Sikorska M. Topoisomerase II-reactive chemotherapeutic drugs induce apoptosis in thymocytes. Cancer Res. 1991 Feb 15;51(4):1078–1085. [PubMed] [Google Scholar]
- Yokomizo A., Ono M., Nanri H., Makino Y., Ohga T., Wada M., Okamoto T., Yodoi J., Kuwano M., Kohno K. Cellular levels of thioredoxin associated with drug sensitivity to cisplatin, mitomycin C, doxorubicin, and etoposide. Cancer Res. 1995 Oct 1;55(19):4293–4296. [PubMed] [Google Scholar]
- van Maanen J. M., Lafleur M. V., Mans D. R., van den Akker E., de Ruiter C., Kootstra P. R., Pappie D., de Vries J., Retèl J., Pinedo H. M. Effects of the ortho-quinone and catechol of the antitumor drug VP-16-213 on the biological activity of single-stranded and double-stranded phi X174 DNA. Biochem Pharmacol. 1988 Oct 1;37(19):3579–3589. doi: 10.1016/0006-2952(88)90388-7. [DOI] [PubMed] [Google Scholar]
- van Maanen J. M., Retèl J., de Vries J., Pinedo H. M. Mechanism of action of antitumor drug etoposide: a review. J Natl Cancer Inst. 1988 Dec 7;80(19):1526–1533. doi: 10.1093/jnci/80.19.1526. [DOI] [PubMed] [Google Scholar]
- van Maanen J. M., de Vries J., Pappie D., van den Akker E., Lafleur V. M., Retèl J., van der Greef J., Pinedo H. M. Cytochrome P-450-mediated O-demethylation: a route in the metabolic activation of etoposide (VP-16-213). Cancer Res. 1987 Sep 1;47(17):4658–4662. [PubMed] [Google Scholar]



