Abstract
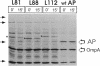
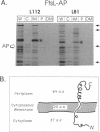
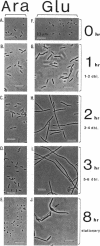
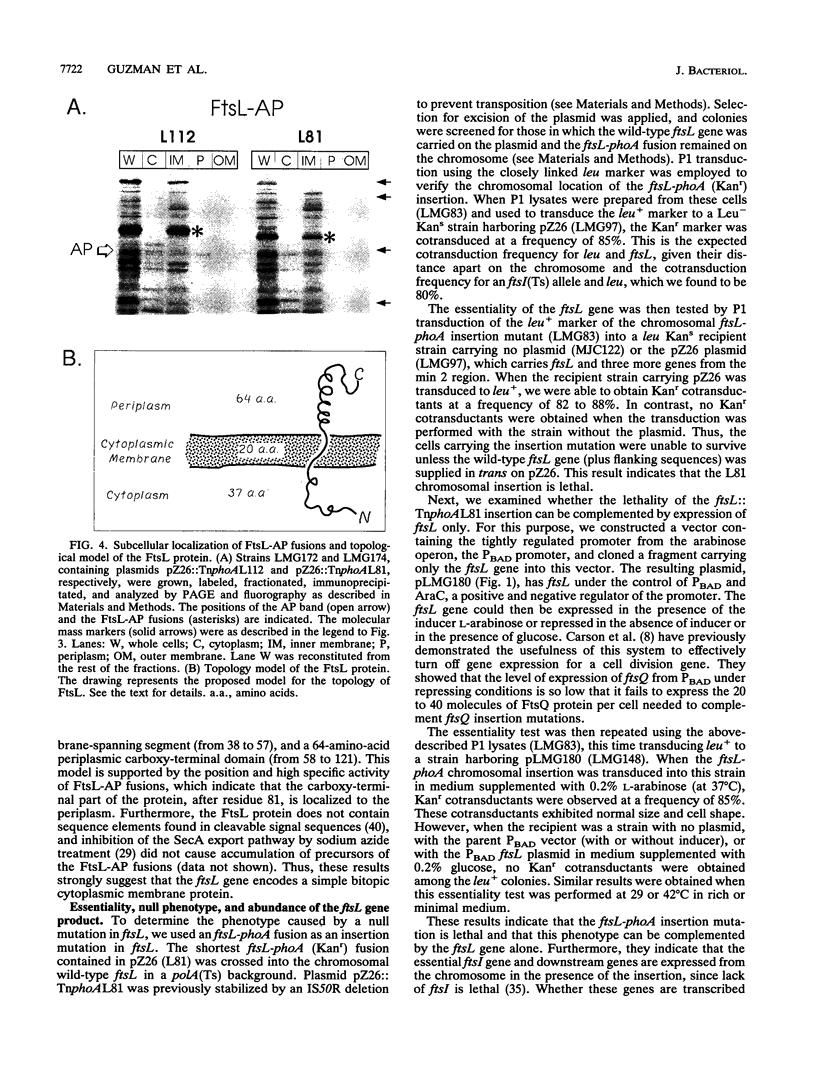
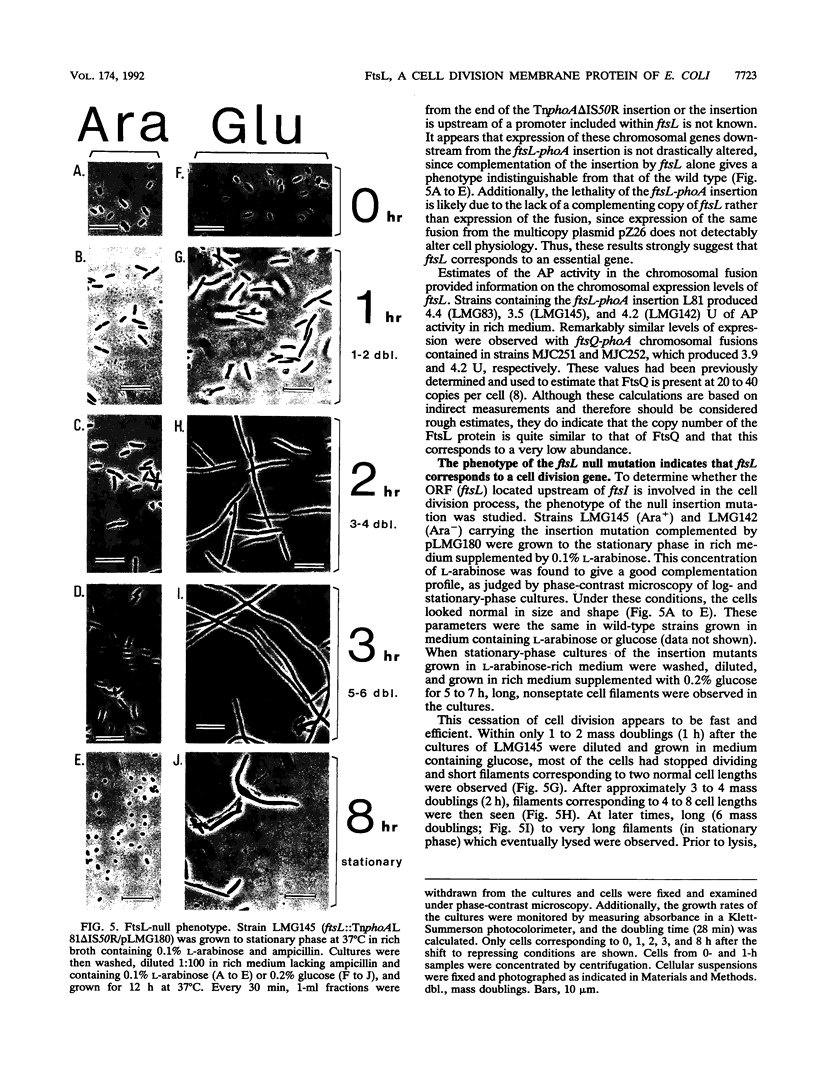
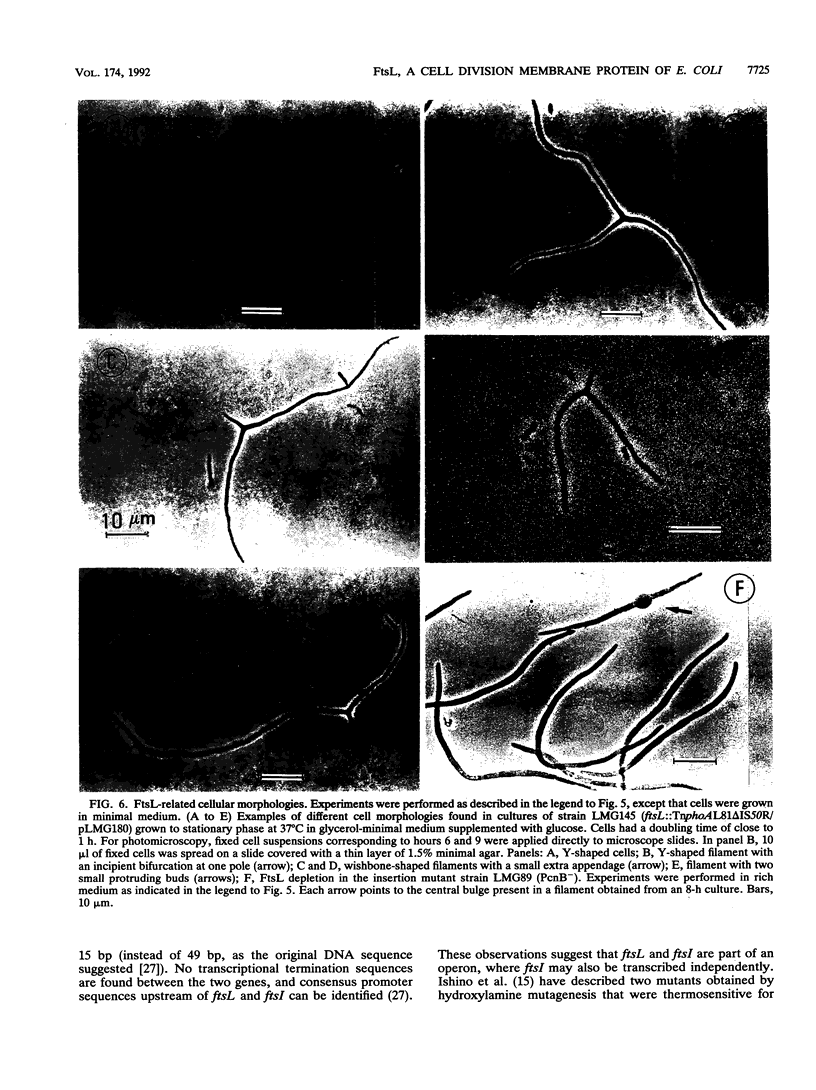
We have identified a gene involved in bacterial cell division, located immediately upstream of the ftsI gene in the min 2 region of the Escherichia coli chromosome. This gene, which we named ftsL, was detected through characterization of TnphoA insertions in a plasmid containing this chromosomal region. TnphoA topological analysis and fractionation of alkaline phosphatase fusion proteins indicated that the ftsL gene product is a 13.6-kDa cytoplasmic membrane protein with a cytoplasmic amino terminus, a single membrane-spanning segment, and a periplasmic carboxy terminus. The ftsL gene is essential for cell growth and division. A null mutation in ftsL resulted in inhibition of cell division, formation of long, nonseptate filaments, ultimate cessation of growth, and lysis. Under certain growth conditions, depletion of FtsL or expression of the largest ftsL-phoA fusion produced a variety of cell morphologies, including Y-shaped bacteria, indicating a possible general weakening of the cell wall. The FtsL protein is estimated to be present at about 20 to 40 copies per cell. The periplasmic domain of the protein displays a sequence with features characteristic of leucine zippers, which are involved in protein dimerization.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldea M., Hernández-Chico C., de la Campa A. G., Kushner S. R., Vicente M. Identification, cloning, and expression of bolA, an ftsZ-dependent morphogene of Escherichia coli. J Bacteriol. 1988 Nov;170(11):5169–5176. doi: 10.1128/jb.170.11.5169-5176.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barondess J. J., Carson M., Guzman Verduzco L. M., Beckwith J. Alkaline phosphatase fusions in the study of cell division genes. Res Microbiol. 1991 Feb-Apr;142(2-3):295–299. doi: 10.1016/0923-2508(91)90044-b. [DOI] [PubMed] [Google Scholar]
- Begg K. J., Donachie W. D. Cell shape and division in Escherichia coli: experiments with shape and division mutants. J Bacteriol. 1985 Aug;163(2):615–622. doi: 10.1128/jb.163.2.615-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E. F., Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991 Nov 14;354(6349):161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- Bi E., Lutkenhaus J. Isolation and characterization of ftsZ alleles that affect septal morphology. J Bacteriol. 1992 Aug;174(16):5414–5423. doi: 10.1128/jb.174.16.5414-5423.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel A., Bedouelle H. Engineering the quaternary structure of an exported protein with a leucine zipper. Protein Eng. 1991 Apr;4(4):457–461. doi: 10.1093/protein/4.4.457. [DOI] [PubMed] [Google Scholar]
- Bowler L. D., Spratt B. G. Membrane topology of penicillin-binding protein 3 of Escherichia coli. Mol Microbiol. 1989 Sep;3(9):1277–1286. doi: 10.1111/j.1365-2958.1989.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Boyd D., Manoil C., Beckwith J. Determinants of membrane protein topology. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8525–8529. doi: 10.1073/pnas.84.23.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson M. J., Barondess J., Beckwith J. The FtsQ protein of Escherichia coli: membrane topology, abundance, and cell division phenotypes due to overproduction and insertion mutations. J Bacteriol. 1991 Apr;173(7):2187–2195. doi: 10.1128/jb.173.7.2187-2195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopazo A., Palacios P., Sánchez M., Pla J., Vicente M. An amino-proximal domain required for the localization of FtsQ in the cytoplasmic membrane, and for its biological function in Escherichia coli. Mol Microbiol. 1992 Mar;6(6):715–722. doi: 10.1111/j.1365-2958.1992.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Guzmán-Verduzco L. M., Kupersztoch Y. M. Export and processing analysis of a fusion between the extracellular heat-stable enterotoxin and the periplasmic B subunit of the heat-labile enterotoxin in Escherichia coli. Mol Microbiol. 1990 Feb;4(2):253–264. doi: 10.1111/j.1365-2958.1990.tb00592.x. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Ishino F., Jung H. K., Ikeda M., Doi M., Wachi M., Matsuhashi M. New mutations fts-36, lts-33, and ftsW clustered in the mra region of the Escherichia coli chromosome induce thermosensitive cell growth and division. J Bacteriol. 1989 Oct;171(10):5523–5530. doi: 10.1128/jb.171.10.5523-5530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino F., Matsuhashi M. Peptidoglycan synthetic enzyme activities of highly purified penicillin-binding protein 3 in Escherichia coli: a septum-forming reaction sequence. Biochem Biophys Res Commun. 1981 Aug 14;101(3):905–911. doi: 10.1016/0006-291x(81)91835-0. [DOI] [PubMed] [Google Scholar]
- Jones C., Holland I. B. Role of the SulB (FtsZ) protein in division inhibition during the SOS response in Escherichia coli: FtsZ stabilizes the inhibitor SulA in maxicells. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6045–6049. doi: 10.1073/pnas.82.18.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow M., Fayet O., Cegielska A., Ziegelhoffer T., Georgopoulos C. Isolation and characterization of the Escherichia coli htrB gene, whose product is essential for bacterial viability above 33 degrees C in rich media. J Bacteriol. 1991 Jan;173(2):741–750. doi: 10.1128/jb.173.2.741-750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopilato J., Bortner S., Beckwith J. Mutations in a new chromosomal gene of Escherichia coli K-12, pcnB, reduce plasmid copy number of pBR322 and its derivatives. Mol Gen Genet. 1986 Nov;205(2):285–290. doi: 10.1007/BF00430440. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. Regulation of cell division in E. coli. Trends Genet. 1990 Jan;6(1):22–25. doi: 10.1016/0168-9525(90)90045-8. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Manoil C., Mekalanos J. J., Beckwith J. Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol. 1990 Feb;172(2):515–518. doi: 10.1128/jb.172.2.515-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxon M. E., Wigboldus J., Brot N., Weissbach H. Structure-function studies on Escherichia coli MetR protein, a putative prokaryotic leucine zipper protein. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7076–7079. doi: 10.1073/pnas.87.18.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Inouye H., Oliver D., Beckwith J. Mutations that alter the signal sequence of alkaline phosphatase in Escherichia coli. J Bacteriol. 1983 Apr;154(1):366–374. doi: 10.1128/jb.154.1.366-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T., Matsuzawa H., Matsuhashi M., Sugino Y. Cell wall peptidoglycan mutants of Escherichia coli K-12: existence of two clusters of genes, mra and mrb, for cell wall peptidoglycan biosynthesis. J Bacteriol. 1972 Nov;112(2):950–958. doi: 10.1128/jb.112.2.950-958.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Donachie W. D. Differential translation of cell division proteins. J Bacteriol. 1990 Oct;172(10):6106–6111. doi: 10.1128/jb.172.10.6106-6111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Maruyama I. N., Soma M., Kato J., Suzuki H., Horota Y. On the process of cellular division in Escherichia coli: nucleotide sequence of the gene for penicillin-binding protein 3. Mol Gen Genet. 1983;191(1):1–9. doi: 10.1007/BF00330881. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Takeda Y., Nishimura A., Suzuki H., Inouye M., Hirota Y. Synthetic ColE1 plasmids carrying genes for cell division in Escherichia coli. Plasmid. 1977 Nov;1(1):67–77. doi: 10.1016/0147-619x(77)90009-9. [DOI] [PubMed] [Google Scholar]
- O'Shea E. K., Rutkowski R., Kim P. S. Evidence that the leucine zipper is a coiled coil. Science. 1989 Jan 27;243(4890):538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- Robinson A. C., Kenan D. J., Hatfull G. F., Sullivan N. F., Spiegelberg R., Donachie W. D. DNA sequence and transcriptional organization of essential cell division genes ftsQ and ftsA of Escherichia coli: evidence for overlapping transcriptional units. J Bacteriol. 1984 Nov;160(2):546–555. doi: 10.1128/jb.160.2.546-555.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. Role of eukaryotic-type functional domains found in the prokaryotic enhancer receptor factor sigma 54. Cell. 1990 Sep 7;62(5):945–954. doi: 10.1016/0092-8674(90)90269-k. [DOI] [PubMed] [Google Scholar]
- Satta G., Fontana R. Characterization of a conditional mutant with altered envelope showing pH-dependent morphology and temperature-dependent division. J Gen Microbiol. 1974 Jan;80(1):51–63. doi: 10.1099/00221287-80-1-51. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Strauch K. L., Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormo A., Ayala J. A., de Pedro M. A., Aldea M., Vicente M. Interaction of FtsA and PBP3 proteins in the Escherichia coli septum. J Bacteriol. 1986 Jun;166(3):985–992. doi: 10.1128/jb.166.3.985-992.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropsha A., Bowen J. P., Brown F. K., Kizer J. S. Do interhelical side chain-backbone hydrogen bonds participate in formation of leucine zipper coiled coils? Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9488–9492. doi: 10.1073/pnas.88.21.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tétart F., Albigot R., Conter A., Mulder E., Bouché J. P. Involvement of FtsZ in coupling of nucleoid separation with septation. Mol Microbiol. 1992 Mar;6(5):621–627. doi: 10.1111/j.1365-2958.1992.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Ueki M., Wachi M., Jung H. K., Ishino F., Matsuhashi M. Escherichia coli mraR gene involved in cell growth and division. J Bacteriol. 1992 Dec;174(23):7841–7843. doi: 10.1128/jb.174.23.7841-7843.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. D., de Boer P. A., Rothfield L. I. A factor that positively regulates cell division by activating transcription of the major cluster of essential cell division genes of Escherichia coli. EMBO J. 1991 Nov;10(11):3363–3372. doi: 10.1002/j.1460-2075.1991.tb04900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. E., Jr, Lutkenhaus J. Overproduction of FtsZ induces minicell formation in E. coli. Cell. 1985 Oct;42(3):941–949. doi: 10.1016/0092-8674(85)90290-9. [DOI] [PubMed] [Google Scholar]
- Zagursky R. J., Berman M. L. Cloning vectors that yield high levels of single-stranded DNA for rapid DNA sequencing. Gene. 1984 Feb;27(2):183–191. doi: 10.1016/0378-1119(84)90139-2. [DOI] [PubMed] [Google Scholar]
- de Boer P. A., Cook W. R., Rothfield L. I. Bacterial cell division. Annu Rev Genet. 1990;24:249–274. doi: 10.1146/annurev.ge.24.120190.001341. [DOI] [PubMed] [Google Scholar]
- de Boer P. A., Crossley R. E., Rothfield L. I. Roles of MinC and MinD in the site-specific septation block mediated by the MinCDE system of Escherichia coli. J Bacteriol. 1992 Jan;174(1):63–70. doi: 10.1128/jb.174.1.63-70.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. The signal peptide. J Membr Biol. 1990 May;115(3):195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]