Abstract
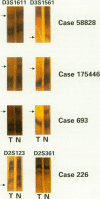
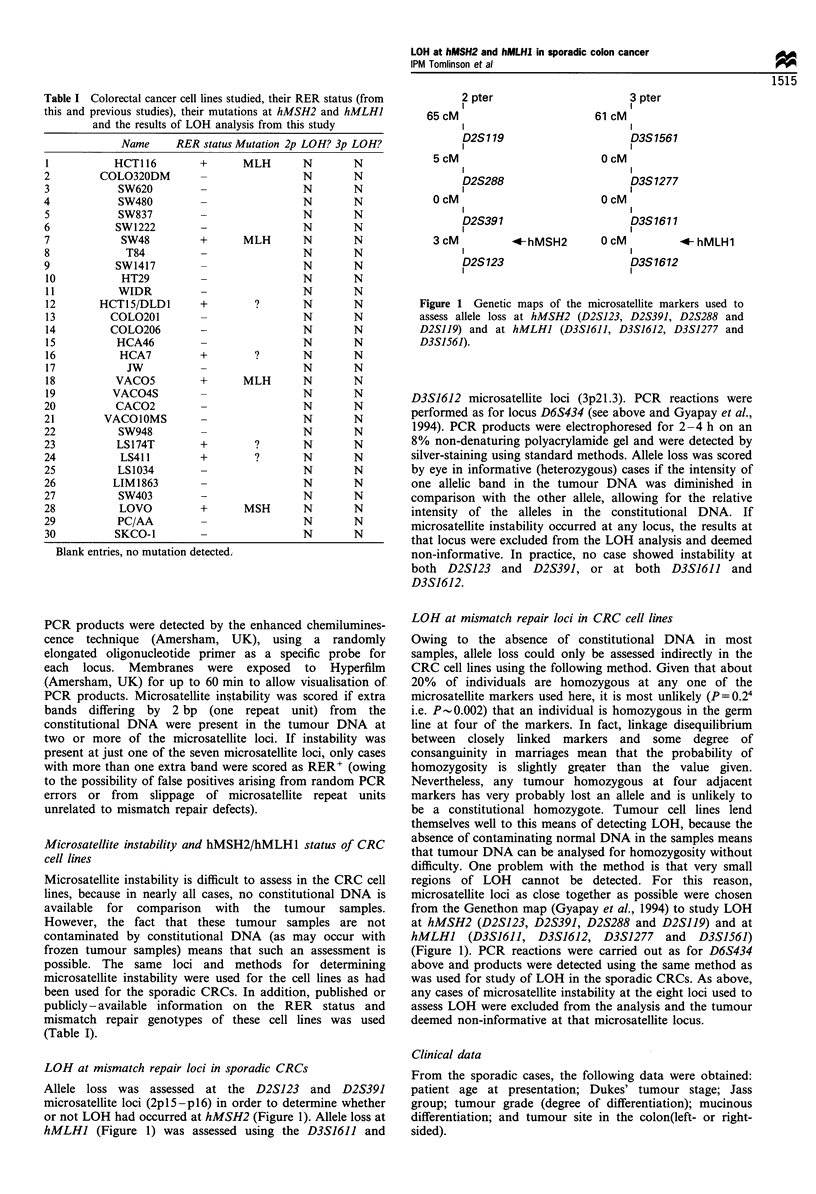
Mutations at the hMSH2 and hMLH1 mismatch repair loci have been implicated in the pathogenesis of colorectal cancer. Tumours with two allelic mutations at a mismatch repair locus develop replication errors (RERs). In the hereditary non-polyposis colorectal cancer (HNPCC) syndrome, one mutation is inherited and the other acquired somatically: in RER+ sporadic colorectal cancers, both mutations are somatic. RER+ tumours tend to have a low frequency of allele loss, presumably because they acquire most mutations through RERs. However, before a second mismatch repair mutation has occurred somatically, there is no reason to suppose that allele loss occurs less frequently in tumours that are to become RER+. Indeed, this second mutation might itself occur by allele loss. We have searched for allele loss at the hMSH2 and hMLH1 loci in RER+ and RER- sporadic colorectal cancers. Loss occurred at the hMLH1 locus in 7/17 (41%) RER+ tumours, compared with 6/40 (15%) RER- cancers (chi2=3.82, P approximately 0.05). At hMSH2, 2/22 RER+ sporadic cancers (9%) had lost an allele, compared with 2/40 (5%) RER- cancers (chi2=0.03, P>0.5). Taken together with previous studies which focused on colorectal cancers from HNPCC families, the data suggest that allele loss at hMLH1, but not at hMSH2, contributes to defective mismatch repair in inherited and sporadic colorectal cancer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Devilee P., van den Broek M., Mannens M., Slater R., Cornelisse C. J., Westerveld A., Khan P. M. Differences in patterns of allelic loss between two common types of adult cancer, breast and colon carcinoma, and Wilms' tumor of childhood. Int J Cancer. 1991 Apr 1;47(6):817–821. doi: 10.1002/ijc.2910470604. [DOI] [PubMed] [Google Scholar]
- Fishel R., Kolodner R. D. Identification of mismatch repair genes and their role in the development of cancer. Curr Opin Genet Dev. 1995 Jun;5(3):382–395. doi: 10.1016/0959-437x(95)80055-7. [DOI] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Hauge X. Y., Grandy D. K., Eubanks J. H., Evans G. A., Civelli O., Litt M. Detection and characterization of additional DNA polymorphisms in the dopamine D2 receptor gene. Genomics. 1991 Jul;10(3):527–530. doi: 10.1016/0888-7543(91)90431-d. [DOI] [PubMed] [Google Scholar]
- Hemminki A., Peltomäki P., Mecklin J. P., Järvinen H., Salovaara R., Nyström-Lahti M., de la Chapelle A., Aaltonen L. A. Loss of the wild type MLH1 gene is a feature of hereditary nonpolyposis colorectal cancer. Nat Genet. 1994 Dec;8(4):405–410. doi: 10.1038/ng1294-405. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Smyrk T. C., Stewart S. M., Lane M. R., Lanspa S. J., Lynch H. T. Pathology of hereditary non-polyposis colorectal cancer. Anticancer Res. 1994 Jul-Aug;14(4B):1631–1634. [PubMed] [Google Scholar]
- Liu B., Nicolaides N. C., Markowitz S., Willson J. K., Parsons R. E., Jen J., Papadopolous N., Peltomäki P., de la Chapelle A., Hamilton S. R. Mismatch repair gene defects in sporadic colorectal cancers with microsatellite instability. Nat Genet. 1995 Jan;9(1):48–55. doi: 10.1038/ng0195-48. [DOI] [PubMed] [Google Scholar]
- Peltomäki P. Microsatellite instability and hereditary non-polyposis colon cancer. J Pathol. 1995 Aug;176(4):329–330. doi: 10.1002/path.1711760402. [DOI] [PubMed] [Google Scholar]
- Radman M., Wagner R. Carcinogenesis. Missing mismatch repair. Nature. 1993 Dec 23;366(6457):722–722. doi: 10.1038/366722a0. [DOI] [PubMed] [Google Scholar]
- Telatar M., Concannon P., Tolun A. Dinucleotide repeat polymorphism at the NCAM locus. Hum Mol Genet. 1994 May;3(5):842–842. doi: 10.1093/hmg/3.5.842. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. P., Bodmer W. F. Chromosome 11q in sporadic colorectal carcinoma: patterns of allele loss and their significance for tumorigenesis. J Clin Pathol. 1996 May;49(5):386–390. doi: 10.1136/jcp.49.5.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnich L., Groenewald I., Theart L., Retief A. E. Highly informative dinucleotide repeat polymorphism at the D11S29 locus on chromosome 11q23. Hum Genet. 1992 May;89(3):357–359. doi: 10.1007/BF00220560. [DOI] [PubMed] [Google Scholar]