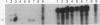Abstract
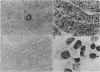
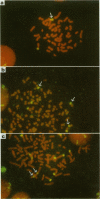
The EVI-1 gene was originally detected as an ectopic viral insertion site and encodes a nuclear zinc finger DNA-binding protein. Previous studies showed restricted EVI-1 RNA or protein expression during ontogeny; in a kidney and an endometrial carcinoma cell line; and in normal murine oocytes and kidney cells. EVI-1 expression was also detected in a subset of acute myeloid leukaemias (AMLs) and myelodysplasia. Because EVI-1 is expressed in the urogenital tract during development, we examined ovarian cancers and normal ovaries for EVI-1 RNA expression using reverse transcription polymerase chain reaction (RT-PCR) and RNAase protection. Chromosome abnormalities were examined using karyotypes and whole chromosome 3 and 3q26 fluorescence in situ hybridisation (FISH). RNA from six primary ovarian tumours, five normal ovaries and 47 tumour cell lines (25 ovarian, seven melanoma, three prostate, seven breast and one each of bladder, endometrial, lung, epidermoid and histiocytic lymphoma) was studied. Five of six primary ovarian tumours, three of five normal ovaries and 22 of 25 ovarian cell lines expressed EVI-1 RNA. A variety of other non-haematological cancers also expressed EVI-1 RNA. Immunostaining of ovarian cancer cell lines revealed nuclear EVI-1 protein. In contrast, normal ovary stained primarily within oocytes and faintly in stroma. Primary ovarian tumours showed nuclear and intense, diffuse cytoplasmic staining. Quantitation of EVI-1 RNA, performed using RNAase protection, showed ovarian carcinoma cells expressed 0 to 40 times the EVI-1 RNA in normal ovary, and 0-6 times the levels in leukaemia cell lines. Southern analyses of ovarian carcinoma cell lines showed no amplification or rearrangements involving EVI-1. In some acute leukaemias, activation of EVI-1 transcription is associated with translocations involving 3q26, the site of the EVI-1 gene. Ovarian carcinoma karyotypes showed one line with quadruplication 3(q24q27), but no other clonal structural rearrangements involving 3q26. However, whole chromsome 3 and 3q26 FISH performed on lines with high EVI-1 expression showed translocations involving chromosome 3q26. EVI-1 is overexpressed in ovarian cancer compared with normal ovaries, suggesting a role for EVI-1 in solid tumour carcinogenesis or progression. Mechanisms underlying EVI-1 overexpression remain unclear, but may include rearrangements involving chromosome 3q26.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew C., Clark A. M. Induction of two alternatively spliced evi-1 proto-oncogene transcripts by cAMP in kidney cells. Oncogene. 1994 Mar;9(3):939–942. [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T., Montgomery S. Cancer statistics, 1994. CA Cancer J Clin. 1994 Jan-Feb;44(1):7–26. doi: 10.3322/canjclin.44.1.7. [DOI] [PubMed] [Google Scholar]
- Buick R. N., Pullano R., Trent J. M. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985 Aug;45(8):3668–3676. [PubMed] [Google Scholar]
- Cheng J. Q., Godwin A. K., Bellacosa A., Taguchi T., Franke T. F., Hamilton T. C., Tsichlis P. N., Testa J. R. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwel R., Funabiki T., Kreider B. L., Morishita K., Ihle J. N. Four of the seven zinc fingers of the Evi-1 myeloid-transforming gene are required for sequence-specific binding to GA(C/T)AAGA(T/C)AAGATAA. Mol Cell Biol. 1993 Jul;13(7):4291–4300. doi: 10.1128/mcb.13.7.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga G., Guenther C., Horvitz H. R. Migrations of the Caenorhabditis elegans HSNs are regulated by egl-43, a gene encoding two zinc finger proteins. Genes Dev. 1993 Nov;7(11):2097–2109. doi: 10.1101/gad.7.11.2097. [DOI] [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Guan X. Y., Cargile C. B., Anzick S. L., Thompson F. H., Meltzer P. S., Bittner M. L., Taetle R., McGill J. R., Trent J. M. Chromosome microdissection identifies cryptic sites of DNA sequence amplification in human ovarian carcinoma. Cancer Res. 1995 Aug 1;55(15):3380–3385. [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Kreider B. L., Orkin S. H., Ihle J. N. Loss of erythropoietin responsiveness in erythroid progenitors due to expression of the Evi-1 myeloid-transforming gene. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6454–6458. doi: 10.1073/pnas.90.14.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden S. L., Cook D. M., Morris J. F., Gashler A., Sukhatme V. P., Rauscher F. J., 3rd Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science. 1991 Sep 27;253(5027):1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- Mann S. C., Andrews P. A., Howell S. B. Modulation of cis-diamminedichloroplatinum(II) accumulation and sensitivity by forskolin and 3-isobutyl-1-methylxanthine in sensitive and resistant human ovarian carcinoma cells. Int J Cancer. 1991 Jul 30;48(6):866–872. doi: 10.1002/ijc.2910480613. [DOI] [PubMed] [Google Scholar]
- Marks J. R., Davidoff A. M., Kerns B. J., Humphrey P. A., Pence J. C., Dodge R. K., Clarke-Pearson D. L., Iglehart J. D., Bast R. C., Jr, Berchuck A. Overexpression and mutation of p53 in epithelial ovarian cancer. Cancer Res. 1991 Jun 1;51(11):2979–2984. [PubMed] [Google Scholar]
- Matsugi T., Morishita K., Ihle J. N. Identification, nuclear localization, and DNA-binding activity of the zinc finger protein encoded by the Evi-1 myeloid transforming gene. Mol Cell Biol. 1990 Mar;10(3):1259–1264. doi: 10.1128/mcb.10.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey D. D., Stone K. R., Wunderli H., Mickey G. H., Paulson D. F. Characterization of a human prostate adenocarcinoma cell line (DU 145) as a monolayer culture and as a solid tumor in athymic mice. Prog Clin Biol Res. 1980;37:67–84. [PubMed] [Google Scholar]
- Milner B. J., Allan L. A., Eccles D. M., Kitchener H. C., Leonard R. C., Kelly K. F., Parkin D. E., Haites N. E. p53 mutation is a common genetic event in ovarian carcinoma. Cancer Res. 1993 May 1;53(9):2128–2132. [PubMed] [Google Scholar]
- Morishita K., Parganas E., Bartholomew C., Sacchi N., Valentine M. B., Raimondi S. C., Le Beau M. M., Ihle J. N. The human Evi-1 gene is located on chromosome 3q24-q28 but is not rearranged in three cases of acute nonlymphocytic leukemias containing t(3;5)(q25;q34) translocations. Oncogene Res. 1990;5(3):221–231. [PubMed] [Google Scholar]
- Morishita K., Parganas E., Douglass E. C., Ihle J. N. Unique expression of the human Evi-1 gene in an endometrial carcinoma cell line: sequence of cDNAs and structure of alternatively spliced transcripts. Oncogene. 1990 Jul;5(7):963–971. [PubMed] [Google Scholar]
- Morishita K., Parganas E., Parham D. M., Matsugi T., Ihle J. N. The Evi-1 zinc finger myeloid transforming gene is normally expressed in the kidney and in developing oocytes. Oncogene. 1990 Sep;5(9):1419–1423. [PubMed] [Google Scholar]
- Morishita K., Parganas E., William C. L., Whittaker M. H., Drabkin H., Oval J., Taetle R., Valentine M. B., Ihle J. N. Activation of EVI1 gene expression in human acute myelogenous leukemias by translocations spanning 300-400 kilobases on chromosome band 3q26. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3937–3941. doi: 10.1073/pnas.89.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita K., Suzukawa K., Taki T., Ihle J. N., Yokota J. EVI-1 zinc finger protein works as a transcriptional activator via binding to a consensus sequence of GACAAGATAAGATAAN1-28 CTCATCTTC. Oncogene. 1995 May 18;10(10):1961–1967. [PubMed] [Google Scholar]
- Mucenski M. L., Taylor B. A., Ihle J. N., Hartley J. W., Morse H. C., 3rd, Jenkins N. A., Copeland N. G. Identification of a common ecotropic viral integration site, Evi-1, in the DNA of AKXD murine myeloid tumors. Mol Cell Biol. 1988 Jan;8(1):301–308. doi: 10.1128/mcb.8.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naredi P., Heath D. D., Enns R. E., Howell S. B. Cross-resistance between cisplatin and antimony in a human ovarian carcinoma cell line. Cancer Res. 1994 Dec 15;54(24):6464–6468. [PubMed] [Google Scholar]
- O'Halloran T. V. Transition metals in control of gene expression. Science. 1993 Aug 6;261(5122):715–725. doi: 10.1126/science.8342038. [DOI] [PubMed] [Google Scholar]
- Oval J., Jones O. W., Montoya M., Taetle R. Characterization of a factor-dependent acute leukemia cell line with translocation (3;3)(q21;q26). Blood. 1990 Oct 1;76(7):1369–1374. [PubMed] [Google Scholar]
- Oval J., Smedsrud M., Taetle R. Expression and regulation of the evi-1 gene in the human factor-dependent leukemia cell line, UCSD/AML1. Leukemia. 1992 May;6(5):446–451. [PubMed] [Google Scholar]
- Perkins A. S., Fishel R., Jenkins N. A., Copeland N. G. Evi-1, a murine zinc finger proto-oncogene, encodes a sequence-specific DNA-binding protein. Mol Cell Biol. 1991 May;11(5):2665–2674. doi: 10.1128/mcb.11.5.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A. S., Mercer J. A., Jenkins N. A., Copeland N. G. Patterns of Evi-1 expression in embryonic and adult tissues suggest that Evi-1 plays an important regulatory role in mouse development. Development. 1991 Feb;111(2):479–487. doi: 10.1242/dev.111.2.479. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Landegent J., Collins C., Fuscoe J., Segraves R., Lucas J., Gray J. Fluorescence in situ hybridization with human chromosome-specific libraries: detection of trisomy 21 and translocations of chromosome 4. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9138–9142. doi: 10.1073/pnas.85.23.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Insogna K. L., Vignery A. M., Stewart A. F., Broadus A. E., D'Souza S. M., Bertolini D. R., Mundy G. R., Rodan G. A. Factors associated with humoral hypercalcemia of malignancy stimulate adenylate cyclase in osteoblastic cells. J Clin Invest. 1983 Oct;72(4):1511–1515. doi: 10.1172/JCI111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin S. C., Finstad C. L., Federici M. G., Scheiner L., Lloyd K. O., Hoskins W. J. Prevalence and significance of HER-2/neu expression in early epithelial ovarian cancer. Cancer. 1994 Mar 1;73(5):1456–1459. doi: 10.1002/1097-0142(19940301)73:5<1456::aid-cncr2820730522>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Rubin S. C., Finstad C. L., Wong G. Y., Almadrones L., Plante M., Lloyd K. O. Prognostic significance of HER-2/neu expression in advanced epithelial ovarian cancer: a multivariate analysis. Am J Obstet Gynecol. 1993 Jan;168(1 Pt 1):162–169. doi: 10.1016/s0002-9378(12)90907-2. [DOI] [PubMed] [Google Scholar]
- Russell M., List A., Greenberg P., Woodward S., Glinsmann B., Parganas E., Ihle J., Taetle R. Expression of EVI1 in myelodysplastic syndromes and other hematologic malignancies without 3q26 translocations. Blood. 1994 Aug 15;84(4):1243–1248. [PubMed] [Google Scholar]
- Russell M., Thompson F., Spier C., Taetle R. Expression of the EVI1 gene in chronic myelogenous leukemia in blast crisis. Leukemia. 1993 Oct;7(10):1654–1657. [PubMed] [Google Scholar]
- Sasano H., Garrett C. T., Wilkinson D. S., Silverberg S., Comerford J., Hyde J. Protooncogene amplification and tumor ploidy in human ovarian neoplasms. Hum Pathol. 1990 Apr;21(4):382–391. doi: 10.1016/0046-8177(90)90199-f. [DOI] [PubMed] [Google Scholar]
- Schleif R. DNA binding by proteins. Science. 1988 Sep 2;241(4870):1182–1187. doi: 10.1126/science.2842864. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Groff D. E., Pokul R. K., Bear J. L., Delgado G. Determination of cellular oncogene rearrangement or amplification in ovarian adenocarcinomas. Am J Obstet Gynecol. 1989 Oct;161(4):911–915. doi: 10.1016/0002-9378(89)90750-3. [DOI] [PubMed] [Google Scholar]
- Taetle R., Honeysett J. M. Effects of monoclonal anti-transferrin receptor antibodies on in vitro growth of human solid tumor cells. Cancer Res. 1987 Apr 15;47(8):2040–2044. [PubMed] [Google Scholar]
- Taetle R., Honeysett J. M., Houston L. L. Effects of anti-epidermal growth factor (EGF) receptor antibodies and an anti-EGF receptor recombinant-ricin A chain immunoconjugate on growth of human cells. J Natl Cancer Inst. 1988 Sep 7;80(13):1053–1059. doi: 10.1093/jnci/80.13.1053. [DOI] [PubMed] [Google Scholar]
- Taetle R., Jones O. W., Honeysett J. M., Abramson I., Bradshaw C., Reid S. Use of nude mouse xenografts as preclinical screens. Characterization of xenograft-derived melanoma cell lines. Cancer. 1987 Oct 15;60(8):1836–1841. doi: 10.1002/1097-0142(19871015)60:8<1836::aid-cncr2820600827>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Taetle R., Oval J., Smedsrud M., Davis C., Gansbacher B. Analysis of granulocyte-macrophage colony-stimulating factor action in differentiating myeloid leukemia cells: treatment with DMSO may reveal a common pathway for growth factor gene regulation. Exp Hematol. 1991 Mar;19(3):213–220. [PubMed] [Google Scholar]
- Tanaka T., Mitani K., Kurokawa M., Ogawa S., Tanaka K., Nishida J., Yazaki Y., Shibata Y., Hirai H. Dual functions of the AML1/Evi-1 chimeric protein in the mechanism of leukemogenesis in t(3;21) leukemias. Mol Cell Biol. 1995 May;15(5):2383–2392. doi: 10.1128/mcb.15.5.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Nishida J., Mitani K., Ogawa S., Yazaki Y., Hirai H. Evi-1 raises AP-1 activity and stimulates c-fos promoter transactivation with dependence on the second zinc finger domain. J Biol Chem. 1994 Sep 30;269(39):24020–24026. [PubMed] [Google Scholar]
- Thompson F. H., Emerson J., Alberts D., Liu Y., Guan X. Y., Burgess A., Fox S., Taetle R., Weinstein R., Makar R. Clonal chromosome abnormalities in 54 cases of ovarian carcinoma. Cancer Genet Cytogenet. 1994 Mar;73(1):33–45. doi: 10.1016/0165-4608(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Thompson F. H., Nelson M. A., Trent J. M., Guan X. Y., Liu Y., Yang J. M., Emerson J., Adair L., Wymer J., Balfour C. Amplification of 19q13.1-q13.2 sequences in ovarian cancer. G-band, FISH, and molecular studies. Cancer Genet Cytogenet. 1996 Mar;87(1):55–62. doi: 10.1016/0165-4608(95)00248-0. [DOI] [PubMed] [Google Scholar]
- Warrell R. P., Jr, Coonley C. J., Straus D. J., Young C. W. Treatment of patients with advanced malignant lymphoma using gallium nitrate administered as a seven-day continuous infusion. Cancer. 1983 Jun 1;51(11):1982–1987. doi: 10.1002/1097-0142(19830601)51:11<1982::aid-cncr2820511104>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Wilding G., Gelmann E. P., Freter C. E. Phosphoinositide metabolism in human prostate cancer cells in vitro. Prostate. 1990;16(1):15–27. doi: 10.1002/pros.2990160103. [DOI] [PubMed] [Google Scholar]
- Woods L. K., Morgan R. T., Quinn L. A., Moore G. E., Semple T. U., Stedman K. E. Comparison of four new cell lines from patients with adenocarcinoma of the ovary. Cancer Res. 1979 Nov;39(11):4449–4459. [PubMed] [Google Scholar]
- van 't Veer L. J., Hermens R., van den Berg-Bakker L. A., Cheng N. C., Fleuren G. J., Bos J. L., Cleton F. J., Schrier P. I. ras oncogene activation in human ovarian carcinoma. Oncogene. 1988 Feb;2(2):157–165. [PubMed] [Google Scholar]