Abstract
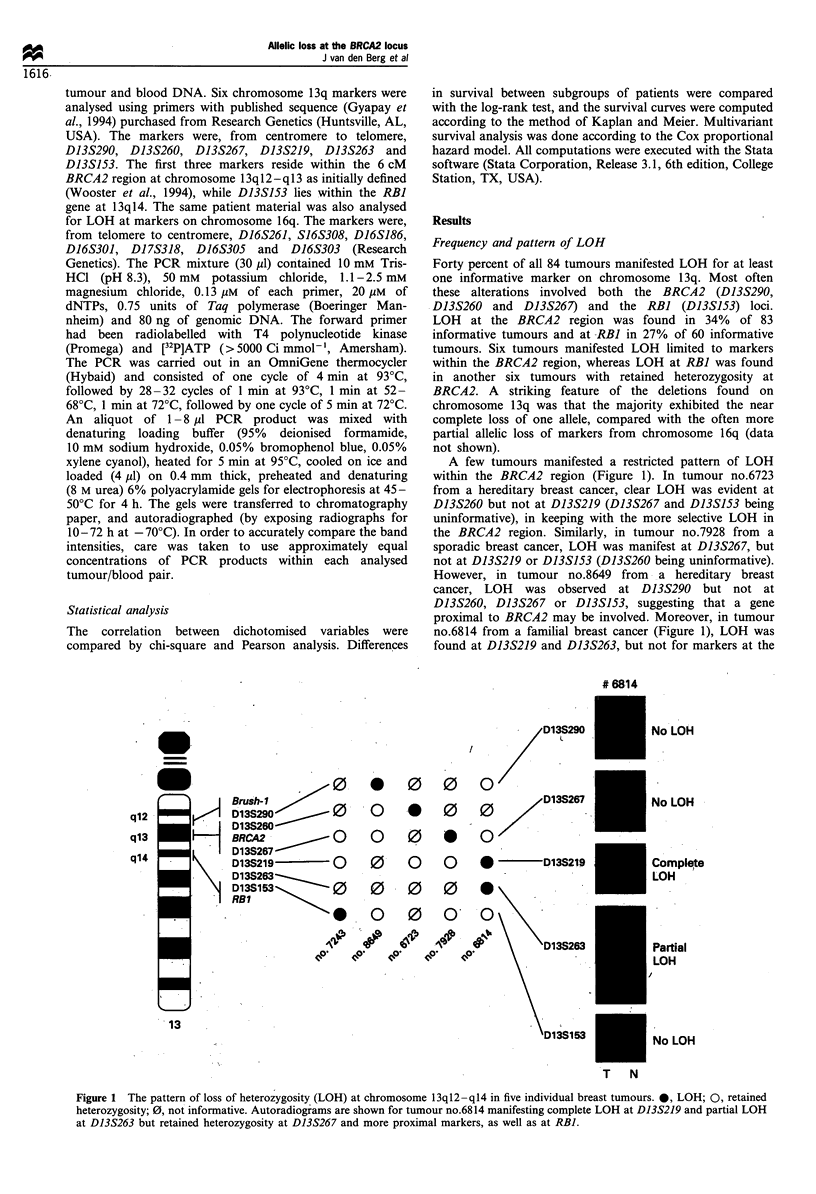
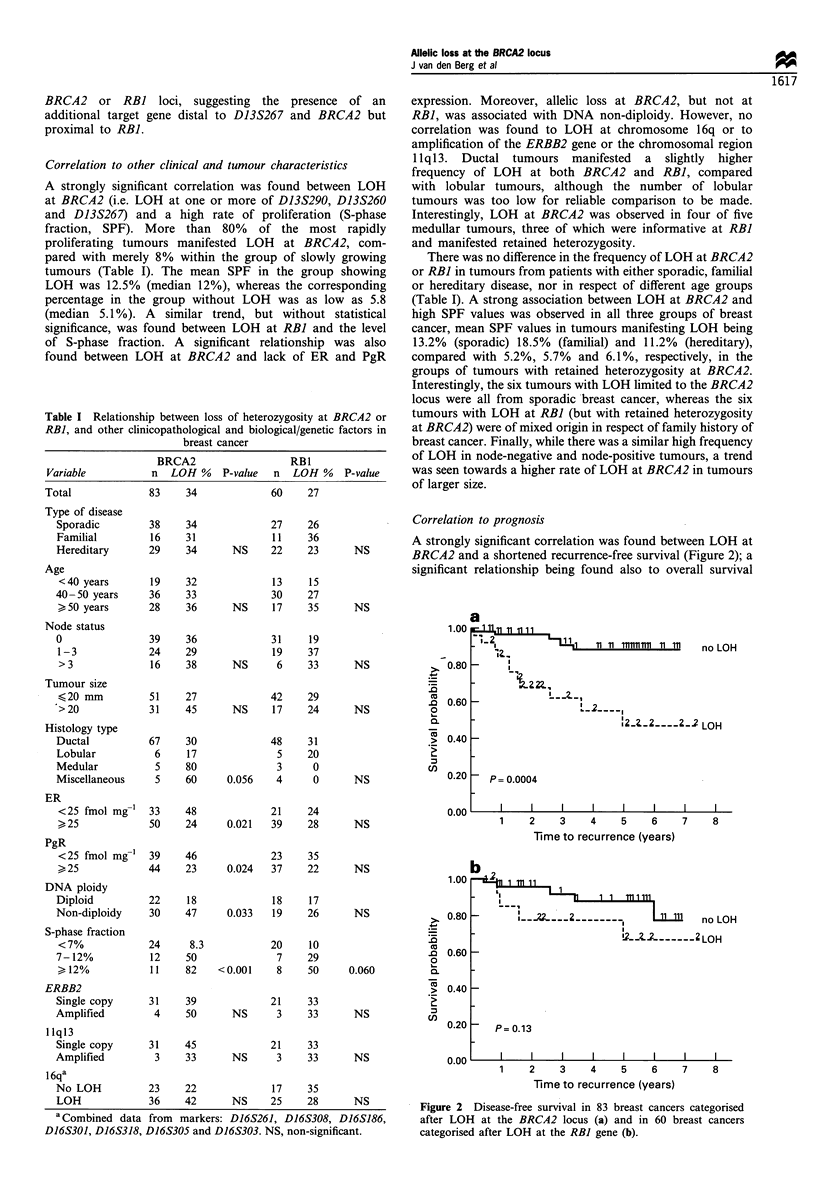
Loss of heterozygosity (LOH) was analysed in 84 primary tumours from sporadic, familial and hereditary breast cancer using five microsatellite markers spanning the chromosomal region 13q12-q13 which harbours the BRCA2 breast cancer susceptibility gene, and using one other marker located within the RBI tumour-suppressor gene at 13q14. LOH at the BRCA2 region was found in 34% and at RBI in 27% of the tumours. Selective LOH at BRCA2 occurred in 7% of the tumours, whereas selective LOH at RBI was observed in another 7%. Moreover, a few tumours demonstrated a restricted deletion pattern, suggesting the presence of additional tumour-suppressor genes both proximal and distal of BRCA2. LOH at BRCA2 was significantly correlated to high S-phase values, low oestrogen and progesterone receptor content and DNA non-diploidy. LOH at BRCA2 was also associated, albeit non-significantly, with large tumour size and the ductal and medullar histological types. No correlation was found with lymph node status, patient age or a family history of breast cancer. A highly significant and independent correlation existed between LOH at BRCA2 and early recurrence and death. LOH at RBI was not associated with the above mentioned factors or prognosis. The present study does not provide conclusive evidence that BRCA2 is the sole target for deletions at 13q12-q13 in breast tumours. However, the results suggest that inactivation of one or several tumour-suppressor genes in the 13q12-q13 region confer a strong tumour growth potential and poor prognosis in both familial and sporadic breast cancer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borg A., Baldetorp B., Fernö M., Killander D., Olsson H., Sigurdsson H. ERBB2 amplification in breast cancer with a high rate of proliferation. Oncogene. 1991 Jan;6(1):137–143. [PubMed] [Google Scholar]
- Borg A., Zhang Q. X., Alm P., Olsson H., Sellberg G. The retinoblastoma gene in breast cancer: allele loss is not correlated with loss of gene protein expression. Cancer Res. 1992 May 15;52(10):2991–2994. [PubMed] [Google Scholar]
- Cleton-Jansen A. M., Collins N., Lakhani S. R., Weissenbach J., Devilee P., Cornelisse C. J., Stratton M. R. Loss of heterozygosity in sporadic breast tumours at the BRCA2 locus on chromosome 13q12-q13. Br J Cancer. 1995 Nov;72(5):1241–1244. doi: 10.1038/bjc.1995.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins N., McManus R., Wooster R., Mangion J., Seal S., Lakhani S. R., Ormiston W., Daly P. A., Ford D., Easton D. F. Consistent loss of the wild type allele in breast cancers from a family linked to the BRCA2 gene on chromosome 13q12-13. Oncogene. 1995 Apr 20;10(8):1673–1675. [PubMed] [Google Scholar]
- Devilee P., van den Broek M., Kuipers-Dijkshoorn N., Kolluri R., Khan P. M., Pearson P. L., Cornelisse C. J. At least four different chromosomal regions are involved in loss of heterozygosity in human breast carcinoma. Genomics. 1989 Oct;5(3):554–560. doi: 10.1016/0888-7543(89)90023-2. [DOI] [PubMed] [Google Scholar]
- Fernö M., Baldetorp B., Borg A., Olsson H., Sigurdsson H., Killander D. Flow cytometric DNA index and S-phase fraction in breast cancer in relation to other prognostic variables and to clinical outcome. Acta Oncol. 1992;31(2):157–165. doi: 10.3109/02841869209088897. [DOI] [PubMed] [Google Scholar]
- Gudmundsson J., Johannesdottir G., Bergthorsson J. T., Arason A., Ingvarsson S., Egilsson V., Barkardottir R. B. Different tumor types from BRCA2 carriers show wild-type chromosome deletions on 13q12-q13. Cancer Res. 1995 Nov 1;55(21):4830–4832. [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Johannsson O., Ostermeyer E. A., Håkansson S., Friedman L. S., Johansson U., Sellberg G., Brøndum-Nielsen K., Sele V., Olsson H., King M. C. Founding BRCA1 mutations in hereditary breast and ovarian cancer in southern Sweden. Am J Hum Genet. 1996 Mar;58(3):441–450. [PMC free article] [PubMed] [Google Scholar]
- Kerangueven F., Allione F., Noguchi T., Adélaïde J., Sobol H., Jacquemier J., Birnbaum D. Patterns of loss of heterozygosity at loci from chromosome arm 13q suggests a possible involvement of BRCA2 in sporadic breast tumors. Genes Chromosomes Cancer. 1995 Aug;13(4):291–294. doi: 10.1002/gcc.2870130410. [DOI] [PubMed] [Google Scholar]
- Merajver S. D., Pham T. M., Caduff R. F., Chen M., Poy E. L., Cooney K. A., Weber B. L., Collins F. S., Johnston C., Frank T. S. Somatic mutations in the BRCA1 gene in sporadic ovarian tumours. Nat Genet. 1995 Apr;9(4):439–443. doi: 10.1038/ng0495-439. [DOI] [PubMed] [Google Scholar]
- Schott D. R., Chang J. N., Deng G., Kurisu W., Kuo W. L., Gray J., Smith H. S. A candidate tumor suppressor gene in human breast cancers. Cancer Res. 1994 Mar 15;54(6):1393–1396. [PubMed] [Google Scholar]
- Tavtigian S. V., Simard J., Rommens J., Couch F., Shattuck-Eidens D., Neuhausen S., Merajver S., Thorlacius S., Offit K., Stoppa-Lyonnet D. The complete BRCA2 gene and mutations in chromosome 13q-linked kindreds. Nat Genet. 1996 Mar;12(3):333–337. doi: 10.1038/ng0396-333. [DOI] [PubMed] [Google Scholar]
- Thompson M. E., Jensen R. A., Obermiller P. S., Page D. L., Holt J. T. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet. 1995 Apr;9(4):444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- Varley J. M., Armour J., Swallow J. E., Jeffreys A. J., Ponder B. A., T'Ang A., Fung Y. K., Brammar W. J., Walker R. A. The retinoblastoma gene is frequently altered leading to loss of expression in primary breast tumours. Oncogene. 1989 Jun;4(6):725–729. [PubMed] [Google Scholar]



