Abstract
Narcotic bowel syndrome (NBS) is a subset of opioid bowel dysfunction that is characterized by chronic or frequently recurring abdominal pain that worsens with continued or escalating dosages of narcotics. This syndrome is under recognized and may be becoming more prevalent. This may be due in the United States to increases in using narcotics for chronic non-malignant painful disorders, and the development of maladaptive therapeutic interactions around its use. NBS can occur in patients with no prior gastrointestinal disorder who receive high dosages of narcotics after surgery or acute painful problems, among patients with functional GI disorders or other chronic gastrointestinal diseases who are managed by physicians unaware of the hyperalgesic effects of chronic opioids. The evidence for the enhanced pain perception is based on: a) activation of excitatory anti-analgesic pathways within a bimodal opioid regulation system, b) descending facilitation of pain at the Rostral Ventral Medulla and pain facilitation via dynorphin and CCK activation, and c) glial cell activation that produces morphine tolerance and enhances opioid induced pain. Treatment involves early recognition of the syndrome, an effective physician patient relationship, graded withdrawal of the narcotic according to a specified withdrawal program and the institution of medications to reduce withdrawal effects.
INTRODUCTION
It has long been recognized that opiates affect gastrointestinal motility. These effects, known as opioid bowel (or gastrointestinal) dysfunction are manifest as constipation, nausea, bloating, ileus and sometimes pain (1–3). When pain is the predominant symptom, the condition has been termed narcotic bowel syndrome (NBS). NBS is characterized by the progressive and somewhat paradoxical increase in abdominal pain despite continued or escalating dosages of narcotics prescribed in an effort to relieve the pain. This entity (4–6) was first reported two decades ago in the United States and 10 years ago in China (7). At the UNC Center for Functional Gastrointestinal (GI) and Motility Disorders (www.med.unc.edu\ibs), patients are frequently seen with chronic and refractory gastrointestinal disorders. Many of these patients are experiencing the NBS and benefit from narcotic detoxification.
In this narrative review we discuss our experience with the clinical features of this syndrome, discuss the changing practice of narcotic usage for functional GI pain which may make NBS more common, review new information on the possible neurophysiological determinants of the syndrome, offer diagnostic criteria and recommend an approach to management of patients with NBS. We performed a Medline search and could only identify 4 case reports on this topic spanning over 20 years. Accordingly, there is a limited and fragmented evidence base and the references provide supportive evidence for the statements made based on clinical experience. Nevertheless we consider this to be a rapidly emerging clinical issue that requires attention. We propose that if the physician recognizes the many facets of NBS with proper diagnosis and management the clinical outcome can greatly improve and health care costs may be reduced.
DIAGNOSIS
The syndrome is characterized by chronic or intermittent colicky abdominal pain that worsens when the narcotic effect wears down. While narcotics may seem helpful at first, over time the pain-free periods become shorter and tachyphylaxis occurs, leading to increasing narcotic doses. Ultimately, increasing dosages enhance the adverse effects on pain sensation and delayed motility, thereby initiating the development of NBS.
Although pain is the dominant feature, nausea, bloating, intermittent vomiting, abdominal distension and constipation are common. Eating can aggravate the symptoms, so when the condition lasts for weeks, mild weight loss may occur due to anorexia or a willful restriction of eating out of fear of aggravating the pain (sitophobia). The symptoms may correlate with delayed gastric emptying and intestinal transit.
A common and misleading consequence of NBS is that abdominal X-rays may show signs suggestive of a partial intestinal obstruction, which in fact is due to an adynamic ileus or pseudo-obstruction. There may also be large amounts of fecal retention seen. Laboratory tests including blood count, amylase, lipase, liver chemistries and urinalysis are usually normal.
The key to the diagnosis of NBS is the recognition that chronic or escalating doses of narcotics lead to continued or worsening symptoms rather than benefit. However since the symptoms are nonspecific (4–6), and many clinicians are unaware that narcotic medications can actually sensitize patients to the experience of pain. Thus, continued treatment with narcotics lead to a vicious cycle of pain, use of more narcotics and continued or worsening pain. It is not uncommon for patients to be hospitalized for weeks at a time until they are urged to leave with a prescription for oral narcotics only to visit the emergency room or be readmitted for the pain several days later. Table 1 provides the proposed criteria for the diagnosis of NBS.
Table 1.
Diagnostic Criteria for Narcotic Bowel Syndrome
| Chronic or frequently recurring abdominal pain that is treated with acute high dose or chronic narcotics and all of the following: |
|
|
|
|
A patient may have a structural diagnosis (e.g., inflammatory bowel disease, “chronic pancreatitis”) but the character or activity of the disease process is not sufficient to explain the pain.
CLINICAL FEATURES
NBS remains under-recognized due to lack of knowledge about the long-term effects of narcotics as potentiators of visceral pain and motility disturbances, and difficulties in clinically distinguishing abdominal pain that results from, rather than is benefited by narcotics. It may occur among patients with no history of GI symptoms or narcotic use who receive narcotics to treat persistent post-operative or other types of pain.
Case #1 - NBS developing after abdominal laparotomy with no prior history of GI symptoms or narcotic use
A 40 year old lawyer was admitted to UNC Hospitals with acute severe abdominal pain, nausea, vomiting and fever. There was no prior history of GI illness. Because of marked RLQ tenderness and leukocytosis, she went to surgery with a presumptive diagnosis of appendicitis, however the surgery was non-diagnostic. Postoperatively she received up to 40 mg./day of IV morphine sulfate. Two weeks later while still on the surgery service, she developed worsening abdominal pain and obstipation with x-ray evidence of a partial small bowel obstruction. At surgery, she had obstruction from newly developed adhesions and a small portion of bowel was resected with lysis of adhesions. One week postoperatively she developed peritonitis and an anastomotic perforation was repaired, her 3rd operation. By this time she had been hospitalized for 6 weeks and was receiving on average 40–60 mg./day of IV morphine sulphate. Over the next 2 months the patient remained in the hospital receiving 80 mg morphine sulfate/day for severe abdominal pain, and nausea with vomiting. Abdominal x-rays and CT showed small and large bowel dilatation with no evidence for obstruction. By 10 weeks of hospitalization, a diagnosis of narcotic bowel syndrome was made and the morphine was withdrawn completely over 6 days. Clonidine 0.1 mg. po 3/day, lorazepam 0.1 mg. 3/day and desipramine 50 mg. hs was also prescribed, By the time of discharge she reported about 75% reduction in pain symptoms. It took about 1 additional year for the pain symptoms to completely resolve, and they have not recurred.
NBS may also occur in patients with functional GI disorders (FGID) who are unwittingly being prescribed narcotics in an effort to treat the functional GI condition.
CASE #2 NBS in Post-infectious IBS (PI-IBS) (8) with no prior history of narcotic use
A 37 year old female was referred to UNC GI clinic for treatment of chronic abdominal pain, nausea, vomiting and alternating diarrhea and constipation that began after a salmonella infection 15 years earlier. Increasing abdominal pain led to frequent emergency room visits, hospitalizations and 4 abdominal surgeries to diagnose or treat the abdominal pain. In addition, multiple CT scans, barium enemas, colonoscopy and abdominal/pelvic ultrasounds were also negative. Her physicians prescribed increasing dosages of narcotics for pain control over a 2-year period, and when seen in the UNC GI clinic she was taking 90mg/day of morphine sulfate.
The mutual plan was to undertake a controlled withdrawal from narcotics. She unsuccessfully attempted to stop them on her own and was admitted to UNC hospital with continued nausea, vomiting, abdominal pain and diarrhea. Lorazepam 1 mg. 4/day was prescribed during the withdrawal and morphine sulfate was titrated down from 30 mg. to 20 mg 3/day to 10 mg 3/day for two days at a time and then discontinued. A clonidine patch, 0.2mg was applied 2 days prior to stopping the morphine sulfate completely. Desipramine and paroxetine were gradually increased over several weeks. Symptoms were markedly improved at hospital discharge and three months later she remained off narcotics on desipramine 100mg and paroxetine 20 mg. with no complaints of abdominal pain, nausea or vomiting. Mild constipation was managed with polyethylene glycol solution as needed. Nine months later she reported only 3 episodes of mild abdominal pain and diarrhea that was consistent with her IBS. Several years later she graduated from law school and is currently in practice with no reports of abdominal pain.
It is noteworthy that patients with pre-existing FGID may be able to differentiate their more chronic gastrointestinal symptoms from NBS.
CASE #3 – A patient with FGID who developed a different pain due to NBS
A 42 year old female with a history three caesarean deliveries, laparoscopy for lyses of adhesions and irritable bowel syndrome for 23 years, was referred for treatment for increasing pain. The mild and occasional post-prandial cramping became associated over 3 years with another more persistent chronic lower abdominal pain that seemed different from her more typical IBS symptom. The new pain was not relieved by defecation, though there was associated abdominal, bloating nausea, vomiting and depressive symptoms. Notably, during these 3 years her primary care physician prescribed oxycodone 10 mg TID for the pain. Other medications included clonazepam 0.5mg TID and paroxetine 60 mg QD for anxiety and depressive symptoms.
On two occasions she tried to stop the narcotics, but was unsuccessful due to worsening pain. She was referred to UNC gastroenterology clinic for consultation and agreed to withdraw the narcotic. She continued with clonazepam and paroxetine and was placed on a clonidine 0.1 mg 3/day for one week, with the oxycodone titrated to 5 mg. twice a day for one week and discontinued. She then switched from the clonidine patch clonidine pills 0.1mg 3/day. One year later she was remained off narcotics with no reports of abdominal pain.
Patients with unexplained abdominal pain or functional GI Disorders (FGID) (9) are particularly vulnerable to erroneous narcotic prescribing particularly when the symptoms are severe and make urgent requests for pain relief (10) (11;12). Physicians and patients need to understand the FGIDs as bona fide disorders where symptoms are due to increased motor and visceral sensory reactivity to stressors with dysregulation of the brain-gut axis, and a close relationship of the pain to psychosocial distress (13–15). Here, narcotics are contraindicated. As with case #3, physicians may prescribe narcotics because of the patient’s distress, and the belief that with no clear explanation for the pain, there are no other treatment options. Up to 30% of patients with FGIDs experience severe, daily symptoms (16) leading them to frequently visit clinics and emergency rooms for relief and 43% of patients admitted for abdominal pain are discharged from hospitals with no specific explanation for their pain (17).
The NBS is also prevalent among patients with pre-existing chronic GI diseases (e.g., inflammatory bowel disease, pancreatitis, and diverticulitis). Because the pain is easily attributed to the underlying disorder, the physician may feel justified to use narcotics even when the disease is not demonstrated to be active, or the patient’s complaints are out of proportion to the activity of disease. Furthermore, since the adverse effects of the narcotics may be similar to the GI disease when active, the narcotics are inadvertently continued.
Case #4 – NBS in a woman with Crohn’s disease
A 20 year old female with a 16 month history of prescription narcotic abuse for low back pain was receiving methadone 260 mg/day from a pain clinic. She developed constipation and right lower quadrant pain which led to resection of a 6cm right ovarian cystic teratoma. Post operatively the pain and constipation continued and she was discharged from the gynecology service on her previous methadone and also oxycodone with acetaminophen prn for breakthrough pain. She was readmitted to the gynecology service with obstipation. The methadone was tapered to 230 mg/day and she was given enemas and then released. She returned 3 days later with nausea, vomiting, bloating, and right lower quadrant pain that was worse after eating. An abdominal x-ray and abdominal CT scan demonstrated a short segment of terminal ileum thickening and retained colonic fecal material. Narcotics were re-instituted for what was presumed to be pain from the Crohn’s disease and the pain got worse. Colonoscopy showed a non-obstructing terminal ileum that was congested, and ileal biopsies reported mild chronic active ileitis consistent with Crohn’s disease. A small bowel barium study demonstrated approximately 20cm of thickened but non-obstructing terminal ileum with a few proximal skip areas. It was determined by the GI service that although she had Crohn’s disease, the pain pattern was clinically related to the narcotic bowel syndrome.
She was started on methylprednisolone 40 mg/day and mesalamine 4g/day for the Crohn’s disease. The methadone was tapered 10–20% each day over 11 days, Duloxetine 30 mg was started and increased to 60mg/day at one week. Additionally, clonidine 0.1mg 4/day for narcotic withdrawal control, cyclobenzaprine 10 mg. qhs prn for low back pain and lorazepam 1mg q6hrs for anxiety were initiated. By day 11 she was successfully tapered off methadone and had dramatic improvement in the pain. Six months later she reported no limitation in activities and no abdominal or back pain.
Twelve months later she presented with severe iron deficiency anemia, was noncompliant with the Crohn’s disease medications and had relapsed with the NBS by restarting narcotics for recreational use and devoloped obstipation and severe abdominal pain. She was admitted to the medical service and quickly tapered off her methadone over a four day admission and restarted on treatment for the Crohn’s disease. One month later she had gained all the weight she had lost, the anemia improved, there was no abdominal pain and daily bowel movements were normal.
In this complex case, pain was initially thought to be due to an ovarian cyst, though surgery did not relieve the pain. She was then diagnosed with Crohn’s disease and was treated for what was thought to be pain due to Crohn’s. However, retrospective review demonstrated worsening pain symptoms with increased narcotic use. The GI service noted that she had active but non-obstructing disease, so narcotic withdrawal was instituted. The rapid improvement in pain was associated with the withdrawal of narcotics on both admissions. The later relapse of NBS was due to recreational drug use rather than for treatment of abdominal pain or Crohn’s disease and again the pain resolved with detoxification.
When an underlying GI disease is present, the clinician needs to carefully assess activity of the disease relative to the patient’s pain behavior to determine the degree to which they are associated or not. For both FGID and GI diseases, visceral inflammation or injury can increase afferent signals leading to visceral hypersensitivity. In addition, stress, abuse history, or psychiatric co-morbidities can also centrally amplify further the perception of even regulatory visceral signals (18–20). Furthermore (to be discussed) chronic narcotic use by itself can upregulate nociceptive pathways. This understanding may reduce not only the unwarranted use of narcotics but also the ordering of expensive and painful procedures which increase the pursuit of incidental findings not related to the pain including unneeded surgeries as in Case #4 (e.g., cholecystectomy, hysterectomy or lysis of adhesions) (21). The focus should then be directed toward proper management.
Finally, there is the risk that a patient with an inactive medical disease may present with severe pain complaints in order to obtain narcotics.
CASE #5
A 20 year old woman with a history of ulcerative colitis, status post total colectomy and ileal pouch-anal anastomosis (1 year earlier) and ileostomy takedown ( 9 months earlier) presented to a local hospital with severe mid-epigastric abdominal pain, and her usual 4–6 bowel movements/day without blood. A CT scan suggested free fluid in the right upper quadrant and moderate stool in the J-pouch. She was started on narcotics for the pain and referred to UNC hospitals for further evaluation and treatment.
On the surgical service, she received IV morphine for the pain that was increased to 90 mg. per day due to a lack of response. An EGD was normal, and a pouch endoscopy showed congested mucosa in the ileal pouch and multiple 3–4 mm erosions in the ileum, suggesting Crohn’s disease. A video capsule could not be completed due to retention in the stomach along with food debris due to poor motility. An MRI with MRCP was normal, and an endovaginal pelvic ultrasound showed bilateral small ovarian cysts.
On the second week of hospitalization she had an exploratory laparotomy because of continued pain, nausea and vomiting. There was no intra-operative pathology except for adhesions which were lysed. Post-operatively the pain continued and IV and oral narcotic dosages up to 360 mg./day of morphine equivalent was prescribed. She refused the recommendation to see a psychiatric consultant. By 7 weeks, the surgeons felt this to no longer be a surgical problem and with continued severe pain, nausea and vomiting, she was transferred to the medicine service where she successfully underwent a 5 day narcotic withdrawal program that included duloxetine 30 mg. qd, lorazepam 1.0 mg. 3/day, quetiapine 100 mg. hs. and clonidine 0.1 mg. 3/day. Just before she was to leave, the nurses found her to be sequestering syringes and needles under her bed and on one occasion was seen injecting or withdrawing fluid from an IV bag.
After the detoxification the patient stated she still had pain and wanted to go back on narcotics. She was told that the pain she reported was no different than when she was on high dosages of narcotics and that to continue on narcotics would be harmful. She stated she would only leave the hospital if the PICC line remained. After discussion with her family, she agreed to have the line removed and she was scheduled to come back to GI clinic. The day after discharge she saw her family physician and was prescribed oxycodone with acetaminophen for pain. Several days later she was hospitalized at an outside hospital and signed out when the physicians refused to give her narcotics. She then went to another hospital and received narcotics for suspicion of bowel obstruction and was then discharged on oral narcotics. Two weeks later she was seen in the UNC GI clinic; she discontinued the duloxetine because of cost and desipramine was prescribed for pain control. She acknowledged receiving narcotics from other sources but denied taking them for two days. Several months later she was seen in inflammatory bowel disease clinic and denied taking narcotics but a urine toxicology screen was positive.
Although initially the pain was presumed to be related to complications of her surgically treated ulcerative colitis, after several weeks it was recognized to be due to narcotic bowel syndrome. However, treatment was complicated by the patient exhibiting drug seeking behavior. After detoxification was achieved, she was later thought to be obtaining narcotics from other sources, thus making restriction from narcotics no longer possible.
PHYSICIAN-PATIENT BEHAVIORS RELATED TO NARCOTICS
The 5 cases, although different in their clinical presentations, share common features relating to the physician-patient interaction that contributes to the consequence of prescribing escalating dosages of narcotics (See Figure 1). Typically a patient presents to an inpatient or outpatient service or to an emergency room with longstanding and unrelenting abdominal pain with diagnostic evaluations demonstrating no identifiable disorder to focus therapy. Notably for patients with non-malignant pain, it is the non-verbal communication of pain behaviors over all other clinical factors including diagnosis and diagnostic studies, that predict the prescribing of opioids (22). Thus the patient who demonstrates pain and suffering even with negative evaluations is likely to be prescribed opiates. In addition, existing pressures in the health care system to see more patients in less time, to discharge as quickly as possible, to utilize expensive high tech diagnostic and therapeutic tools and to maximize relative value units (RVUs) tend to reduce the attention paid to patient centered assessment and care, effective communication skills and proper decision making (23). As a result, this leads to insufficient information gathering and a confusing understanding of the clinical presentation. The physician may then embark more upon diagnostic algorithms, imaging scans, and full laboratory panels (23;24), which are usually negative. The patient is then discharged from the clinical service or emergency department with no diagnosis or meaningful treatment plan, and because the patient still has pain, with a prescription for narcotics. The difficulty is then compounded when the care is shifted back to the primary care physician because: 1) The accepting physician is not clear on the diagnosis, 2) he or she may feel conflicted with regard to further prescribing the narcotics given the recommendations, 3) subsequent dialog between physician and patient on narcotic use may undercut other treatment options, and 4) the physician may feel unwilling or unable to manage this clinical situation, or may harbor ambivalent feelings toward the patient. The patient in turn not being aware of any other option than to request narcotics for pain relief, feels helpless and possibly angry at the physician when this request is rejected (12).
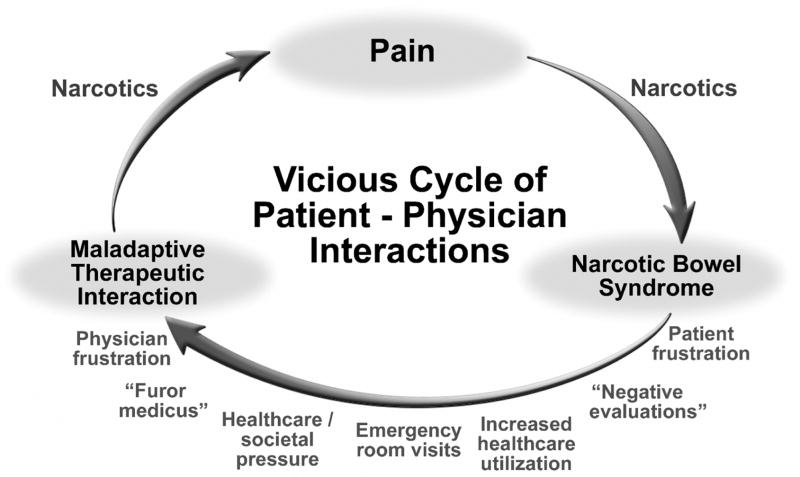
Figure 1.
Vicious cycle of patient-physician interaction in narcotic bowel syndrome. Patient presents with a pain, either due to a structural condition (e.g., IBD), post-surgery or functional GI disorder and narcotics are started. Patient develops symptom of Narcotic Bowel Syndrome. Subsequent evaluation is unrevealing and the narcotics are escalated to treat the abdominal pain with worsening of NBS. This prompts the patient to increase health care utilization or make emergency room visits, which leads to physician frustration and “furor medicusw” leading to a maladaptive therapeutic interaction with additional use of narcotics. The cycle continues until the syndrome is recognized and treatment initiated.
This all-too-common scenario leads to a maladaptive, physician-patient therapeutic relationship along with a failure to treat the condition effectively. This pattern of care may also permit access to the health care system by patients with addictive or malingering behaviors as demonstrated in Case #5. Only a few of such experiences may sensitize the physician to avoid seeing patients with NBS, to react in an angry or defensive manner or just “give up” and indiscriminately prescribe the narcotics. Over time the patient continues to feel dissatisfied and rejected, and may continue maladaptive behaviors with the prescribing physician or seek another physician. This vicious cycle can only be broken when the diagnosis of NBS is made and a narcotic withdrawal program is instituted.
NARCOTIC PRESCRIBING IN THE CURRENT HEALTH CARE SETTING
Impressively, the United States, with 4.6% of the world’s population, uses 80% of the world’s opioids (25). Although treatments with narcotics for these and other conditions should be both controlled and limited, prescriptions are actually increasing over time, and associated with this is an accelerating incidence of narcotic abuse. From 1997 to 2002, there was greater than 400% increase in retail sales of oxycodone and methadone (25). According to the Natinal Institute on Drug Abuse (NIDA, www.drugabuse.gov/Infofacts/nationtreatns.html), there has been a 100% increase in hydrocodone associated emergency room visits over a six year span (1993–1999).
In San Francisco, oxycodone emergency department visits increased 110 percent from 2001–2002. NIDA has indicated that prescription narcotic abuse continues to be on the rise, is widespread around the country, and outpaces other drugs of abuse. NIDA’s Community Epidemiology Work Group (CEWG) report steady increases in oxycodone medical sales, diversion of the drug from clinics, and increased arrests related to this drug. In 11 of 20 national metropolitan CEWG areas in 2001, the number of narcotic analgesic-related death exceeded those for cocaine, heroin/morphine, marijuana, and methamphetamine. Although no data are available, it is probable that, because of these changes, the incidence of NBS is increasing.
There is evidence that the benefit of increasingly using narcotics for non-malignant pain, and particularly for functional GI or chronic GI pain is not as great as previously assumed. A recent systematic literature review found a wide range of methadone dosages prescribed and with lower than expected effectiveness when used in a variety of chronic or non-malignant pain syndromes, and the evidence for benefit was based largely on uncontrolled studies (26). Furthermore, when narcotics are used for functional GI conditions the enhanced sensitivity of the bowel produce greater GI side effects.
This increased use of narcotics with limited benefit and greater risk seems to be occurring due to recent changes in prescribing behaviors. Physicians traditionally prescribed narcotics to younger individuals with acute injuries such as bone fractures, post operative pain, acute severe pain, intermittent painful syndromes (e.g., migraine), and palliative care of malignant conditions. In an aging population, recent practice patterns indicate more prolonged treatments for chronic, non-malignant medical conditions with only limited or no benefit (26). Furthermore, even specialized pain treatment centers have modified their narcotic treatment protocols to include patients with non-malignant pain, including patients with chronic GI disorders. However, even when protocols are used practitioners do not usually adhere to the very policies that they have created (27).
In the 1980’s suggestions were made that low dose narcotics combined with a multi-component pain management program could benefit patients with non-malignant pain and without risk of addiction (28). Unfortunately, this approach has shifted to primary use of more costly rapidly absorbed narcotics administered by inhalation or dermal patch, or given parenterally or intrathecally, at the expense of using the traditional behavioral treatments for chronic non-malignant pain (29). One study of 690 patients demonstrated over-utilization of prescribed narcotics such as oxycodone nearly twice as frequently as indicated (30), despite recent guidelines favoring modalities such as physical and occupational therapy, psychological treatment, and nonnarcotic analgesics (e.g., antidepressants, gabapentin, ketorolac) before employing opiates (31). These other modalities are important since it is recognized that patients with chronic pain report impairments of multiple quality of life measures, including physical social and psychologic well being (31). Analgesics are only a part of the treatment of chronic pain and should be used judiciously so as not to encourage passivity amongst patients to engage in a more complete treatment regimen. (32)
This changing practice pattern is enabled by the greater cost benefit offered by 3rd party payers who support high volume pain clinics that prescribe narcotics or expensive interventions during brief visits. This in turn leads to overutilization of health care resources and increased annual health care expenditures(25), yet there is no evidence that this approach provides any benefit over multimodality therapy, including behavioral treatments and antidepressants (33).
POTENTIAL PHYSIOLOGICAL MECHANISMS FOR PATHOLOGICAL PAIN FACILITATION
It is recognized that morphine and other opiates act on opioid receptors in enteric neurons with a variety of GI effects that includes reduced gastrointestinal and biliary motility and secretion producing nausea, vomiting, constipation, secondary intestinal pseudo-obstruction and gastroparesis (34). Furthermore, the cellular mechanisms for opiate tolerance, i.e., reduced sensitivity to the pharmacological actions of opiates due to chronic exposure are now being uncovered.
Possibly the most perplexing feature of the NBS is to recognize and accept that narcotic analgesics can actually cause or aggravate the very pain that is being treated. This counterintuitive hyperalgesic effect of narcotics relates to the evidence that pain is dynamically modulated by central nervous system (CNS), neural and opioid pathways that can both inhibit and facilitate pain perception, and with chronic opiate use neuroplastic changes appear to occur that paradoxically enhance hyperalgesia and tolerance. Recent studies suggest at least 3 putative mechanisms leading to enhancement of pain experience with the prolonged use of narcotics: a) the existence of a bimodal opioid regulation system where preferential activation of excitatory pathways over time may lead to opiate tolerance and pain augmentation, b) counter-regulatory mechanisms, with release of anti-opioid neuromodulators such as dynorphin and cholecystokinin (CCK) that oppose opioid anti-nociceptive function, and c) glial cell activation that produces morphine tolerance and enhances opiate induced pain.
Bimodal (excitatory and inhibitory) opioid modulation system in the dorsal horn
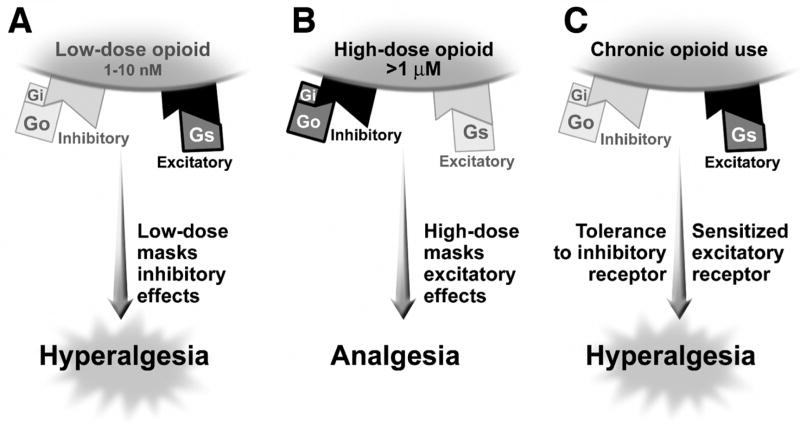
Although activation of opioid receptors is generally considered to produce inhibitory effects on afferent neurons thus reducing afferent signaling, recent studies (35;36) in mice and in vitro indicate that not only can the traditional GI/GO protein inhibitory mode be activated leading to analgesia but also a newly identified GS protein excitatory receptor can be activated to produce hyperalgesia depending in part on the concentration and duration of the opioids acting on the action potential (Figure 2). Thus, there is bimodal (i.e., excitatory and inhibitory) modulation of the action potential of sensory neurons. In the dorsal horn, low concentrations of opioids (1–10 ηM) prolong action potentials producing excitatory effects thereby enhancing neurotransmitter release, while higher concentrations (~1 μM) shorten action potentials thereby inhibiting neurotransmitter release. When these opioids activate the traditionally recognized Gi and Go protein, inhibition of neurotransmission occurs that produces analgesia, and activation of the newly recognized Gs protein activates neurotransmission leading to an excitation mode that produces anti-analgesia and tolerance(36) (Figure 2). The excitatory effects of most opioid agonists have been generally overlooked because they are often masked by the inhibitory effects of the opioids when administered at high (i.e., μM) concentrations used to produce analgesia. However, it is now recognized that Gs-coupled excitatory opioid receptors appear to become progressively sensitized during chronic exposure of dorsal root ganglia to opioid agonists over time leading to tolerance of inhibitory pain effects and ultimately hyperalgesia. For example, in vitro there is reduced inhibitory (via Gi and Go) neurotransmitter effects demonstrated with chronic administration of morphine that is due to the sustained activation of supersensitized excitatory (Gs) opioid receptors (37). These studies also show that low doses of opiate antagonists like naltrexone, naloxone or buprenorphone have selective inhibitory effects on the excitatory (Gs) pathways thus enhancing morphine analgesia(36). Clinically, activation of the excitatory opioid receptor-mediated effects using prolonged high dose narcotic agonists may help explain its adverse effect of producing opioid hyperalgesia and the NBS. Conversely, these observations provide the rationale for the recent release of combination narcotic agonists with low dose narcotic antagonists, the latter serving to block the excitatory pathways, thus enhancing analgesia with lower dosages of narcotics. These agents have successfully been used for narcotic detoxification programs as well. The FDA recently approved Suboxone® (buprenorphine/naloxone) which was evaluated in NIDA’s Center for Clinical Trials Network (CTN). Through this network, two clinical trials in twelve community-based treatment programs assessed Suboxone’s efficacy in short-term opiate detoxification. The clinical trials demonstrated that 68% of patients satisfactorily completed detoxification and in fact, many of these patients had previously failed other detoxification programs. Furthermore, it was found to be safe and well tolerated, even when used by practitioners with minimal experience providing opiate-based pharmacotherapy. (38)
Figure 2.
Bimodal (Excitatory and Inhibitory) Opioid Modulation System in the Dorsal Horn. Opioids appear to have differential effects on the opioid receptor in the dorsal root ganglion based on whether it activates the traditional GI/GO protein inhibitory mode leading to analgesia or a newly identified Gs protein excitatory receptor that can produce hyperalgesia. Figure 2a shows the effect of low dose opioids (1–10 nM), which preferentially activates the excitatory (Gs coupled) mode and masks the inhibitory (Gi/Go) mode leading to hyperalgesia. More typically high dose opioids are used (figure 2b) where there is preferential activation of the inhibitory mode and masking of the excitatory mode. With the chronic use of opioids there is again sensitization and unmasking of the excitatory mode and tolerance of the inhibitory mode leading to hyperalgesia. Low doses of opioid antagonists like naltrexone, naloxone or buprenorphone have selective inhibitory effects on the excitatory pathways which enhances morphine activity.
Descending facilitation of pain at Rostral Ventral Medulla and pain facilitation via dynorphin and CCK activation
Specific regions of the brain, including the cingulate and prefrontal cortex, the Rostral Ventral Medulla (RVM), and periaqueductal gray modulate incoming pain signals at the level of the spinal cord. These areas can produce antinociception via descending inhibitory pathways, effectively attenuating noxious input at the spinal level (39) (18). In addition, the RVM via the dorsolateral funiculus (DVF) can also activate descending tracts that enhance nociceptive input at the spinal cord (40). These responses been demonstrated to occur via activation or inactivation of “on” and “off”cells in the RVM. Activation of the ‘off cells’ produces an inhibition of nociceptive input, while activation of the ‘on cells’ are believed to facilitate nociceptive processing within the RVM and descending projections to the spinal cord (41). Additionally, dynorphin an endogenous opiate when released at the level of the spinal cord is associated with pain syndromes similar to those seen in chronic inflammation and peripheral nerve injury. This is thought to occur via increases in excitatory neurotransmitters from primary afferent neurons, thus provoking a positive feedback loop that amplifies sensory signals (42). Increased dynorphin is also observed in opiate-induced pain states (43) suggesting its role in the pro-nociception process, and in animal models, pain behavior is diminished by administration of anti-serum to dynorphin (43),(44). Sensitization of sensory neurotransmission has also been shown when large doses of spinal morphine are released, leading to paradoxical pronociception, and systemic injection of heroine or morphine can produce a rebound hyperalgesia after the antinociceptive effects have worn off (45). Furthermore, CCK and CCK receptors in the CNS overlap with the distributions of endogenous opioid peptides and receptors and are complementary in modulation of nociception in these descending pathways. (46) (42) and can facilitate descending pain pathways(47) (48).
The involvement of the RVM in this role of paradoxical pain is demonstrated in a study for which opiate withdrawal is initiated by naloxone injection, inducing hyperalgesia and activation of the “on cells,” which can be reversed with lidocaine introduced into the RVM.(49). Thus, these descending pain facilitatory mechanisms influence morphine-induced paradoxical pain and may potentiate the pain experience through these mechanisms.
Effects of glial activation on pathologic pain and facilitation by opioids
The activation of spinal cord glia is a new mechanism that has been found to amplify pathological pain (50). Dorsal horn glia (astrocytes and microglia) when activated produce hyperalgesia in response to inflammation or infection, drugs such as morphine, from peripheral injury, or even in response to signals from the CNS, thus opening the possibility for central effects of stress on peripheral pain facilitation (51). Thus, glial activation can occur from many noxious sources and can further drive neuropathic pain. Conversely blocking glial activation can diminish pain. The effect on pain amplification can occur since the glia cell expresses receptors for many neurotransmitters and neuromodulators, synthesizes and releases numerous transmitters, and is capable of producing proinflammatory cytokines, such as IL-1, IL-6 and TNF (50) all of which can enhance pain transmission and even counter the pain inhibitory effects of morphine.
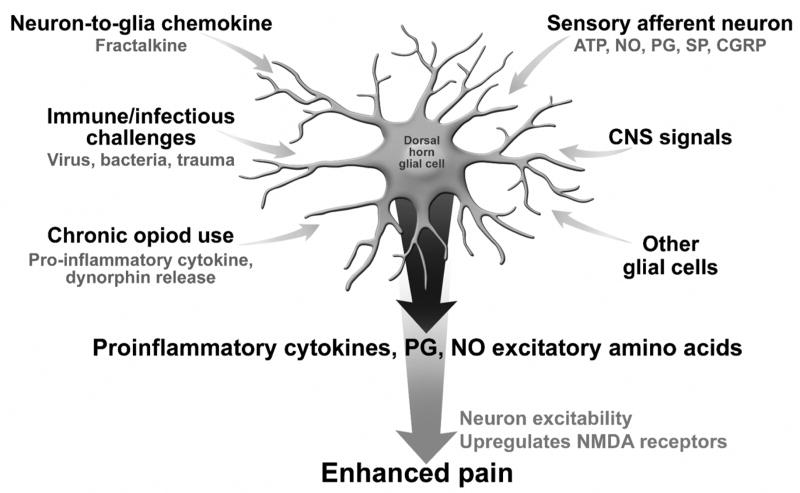
The concept for glia being mediators of hyperalgesia relate to several lines of evidence(51) (Figure 3): a) activated glia release nitric oxide, prostaglandins, excitatory amino acids and growth factors which excite spinal neurons, b) glia can also release substances that enhance the release of pain transmitters from sensory neurons that synapse in the dorsal horn, , c) glia are activated by sensory afferents releasing pain neurotransmitters and neuromodulators (e.g., ATP, substance P, glutamate and calcitonin-gene-related peptide, nitric oxide, and prostaglandins) in the dorsal horn, which in turn lead the glia to perseverate in their nociceptive activity, thus creating a positive feedback loop (50), and finally d) Glia are also activated via a novel neuron-to-glia chemokine, called fractalkine (51). Fractalkine is expressed on extracellular surfaces of spinal neurons, and when neurons are excited, they release bound fractalkine which activates nearby glia (52). Additionally, fractalkine can induce proinflammatory cytokine release from the dorsal spinal cord (53)
Figure 3.
Activation of Dorsal Horn Glial Cells. The activation of spinal cord glial cells leads to hyperalgesia. This process is enabled via stimulation of glial cells to produce proinflammatory cytokines, prostaglandin, nitric oxide and excitatory amino acids, which increase neuron excitability and upregulate NMDA receptors leading to enhanced pain. Factors that activate this mechanism include. : a) release of Adenosin triphosphate (ATP), nitric oxide (NO), prostaglandins, substance P and calcitonin g-related polypeptide (CGRP) from sensory afferent neurons, b) descending signals from the CNS, c) a vicious cycle with activation from other activated glial cells, d) immune challenges or infections, and e) release of a neuron to glia chemokine, fractalkine. Importantly chronic opioid use also activates this system via release of pro-inflammatory cytokines and endogenous dynorphin release. Thus chronic opioid use in addition to these other factors can lead to hyperalgesia via this mechanism.
More recently when using colonic irritation in Sprague-Dawley rats to induce visceral hypersensitivity (54)the introduction of fractalkine, the chemokine specific to neuron to microglia signaling, facilitated EMG responses to noxious colorectal distension and enhanced visceral and somatic nociception. This supports the emerging evidence that microglia are involved in the facilitation of exaggerated nociceptive pain responses to visceral signals.
With regard to the potentiating effect of narcotics on this system, opiates bind directly to glia via μ receptors causing the release of pro-inflammatory cytokines. Opiates can also act indirectly on glia via dynorphin release. Chronic administration of morphine, similar to peripheral nerve injury, increases spinal levels of dynorphin (55) which induces hyperalgesia. Importantly, this glial activation occurs with chronic, but not acute morphine treatment (56). Blocking this effect, reduces hyperalgesia, restores analgesia (57) and prevents development of opiate tolerance.
Although these findings are relatively recent, they help to support the concept of narcotic induced hyperalgesia, clinically recognized over 20 years ago, and raise options for future treatments for chronic pain and the NBS. Further clinical studies are needed to support these proposed mechanisms in humans and pharmacological studies are needed to demonstrate their targeted effects on these receptors that amplify the pain experience.
TREATMENT
Our treatment of NBS as summarized in table 3, involves a biopsychosocial approach. An effective physician patient relationship and a consistent plan of narcotic withdrawal coupled with the initiation of effective alternative treatments to manage the pain and bowel symptoms is recommended. Treatment can be initiated when the diagnosis is made and there is reasonable evidence that no other diagnosis explains the symptoms. NBS is a positive diagnosis which occurs independent of other pathological conditions, and it may also be the cause of pain in patients with existing inactive abdominal pathology. Therefore confirming whether any abdominal pathology is active or inactive is helpful, but an extensive evaluation to “exclude other disease” is not recommended.
Table 3.
Overview of Treatment of Narcotic Bowel Syndrome
|
Physician-patient Relationship
An effective therapeutic relationship is the cornerstone of treatment. However, with patients having NBS, the relationship may be at risk and can become adversarial merely by introducing the plan to withdraw narcotics. From the physician’s perspective the use of narcotics is countertherapeutic, and detoxification could improve the clinical outcome. But from the patient’s perspective, and particularly when no other therapeutic options seem available, narcotics have been prescribed by others, and at least initially helped to reduce the pain. Thus the patient may view the physician who withholds narcotics as either questioning the legitimacy of the symptoms, believing that he or she is addicted, or is rejecting of the patients needs out of the belief that there is little else to offer, or is using the medical position to control the patient’s needs (12). Therefore, it is critical to establish an effective therapeutic relationship before specific treatment recommendations are made. Some approaches to achieving this are available elsewhere (14;58–61). What follows are some specific features of the relationship that are relevant to management.
First, the physician must accept the pain as real and validate the personal impact of the pain using genuine empathy (14;62) (“I can see the pain has really affected your life”). Second the physician needs to provide information through dialog, rather than by lecturing or providing written materials. This includes discussion on the physiological basis for the pain relating to visceral hypersensitivity and the role of brain-gut dysfunction (14;63) in the condition, as well as the difficulties with taking narcotics: “narcotics slow the bowels and produce the constipation, bloating and vomiting you are experiencing,” or “narcotics can over time sensitize the nerves and make the pain worse”. Then the rationale for the treatment plan and the withdrawal of the narcotics can be explained while gauging the patient’s understanding of the information. Here it becomes important to also elicit and respond to the patient’s concerns and expectations about the treatment plan. Although, there is no correct answer to the difficult questions that may emerge, e.g., “How do you know you’re still not missing something”, “What if I get a bad pain attack?,” or “What if these other medicines make me sick” certain responses are in order: a) that the patient has a bona fide disorder that needs to be treated rather spending more money doing studies, but the clinician will stay vigilant to evaluating new findings should they emerge, b) to clarify that the treatment is based on a gradual withdrawal of narcotic as other treatments are substituted, so the patient will not be abandoned in pain, and c) that the physician will be available to address any side effects or flare ups with the patient if they occur.
The next step is to gauge the patient’s willingness to undergo treatment by assessing the genuineness of the response. It is expected that the patient will want to discuss the pros and cons of the treatment plan and to process this information before coming to a decision. A quick acceptance (“anything you say doc”) reflects the patient’s desire to meet physician expectations, or may hide the fact that he or she is not really interested. It is also important to monitor for unrealistic expectations (“Will this make me pain free?” “If it doesn’t work, can I go back on narcotics?”), and then provide reasonable and realistic responses. For example, the physician can state that some patients do become pain free but a more likely effect is that the patient will feel better off the narcotics than when on them, and that this may improve over time as additional treatments start to take effect. The treatment plan should also be discussed with the spouse, parent or other responsible family member, since their understanding of the process and expectations will help them support the patient during difficult periods. Communication that the treatment plan involves ongoing “management” of the painful condition rather than “cure” conveys the physician’s willingness to work with the patient through the ups and downs that the condition may bring about.
Importantly, the treatment does not end with the narcotic withdrawal program. Emotional support through continuity of care is essential, and the physician or the physician extender may need to stay more available for phone calls particularly during the first several weeks after the withdrawal program has ended. It is reasonable to schedule a follow up visit one or two weeks afterwards and then monthly for 2 or 3 months.
Specific Treatment Recommendations
The general approach involves a gradual withdrawal of the narcotic, substituting other treatments that minimize immediate withdrawal effects, treating psychological co-morbidities, and helping to achieve pain control. The recommendations provided are based on clinical experience specifically for patients in our functional GI and motility program, and an example of a withdrawal protocol is presented in Figure 4. It would not apply to habitual users of “recreational” narcotics or those with drug seeking behaviors (e.g., Case #5) where referral to more comprehensive psychiatric or narcotic management programs using other protocols would apply (64;65).
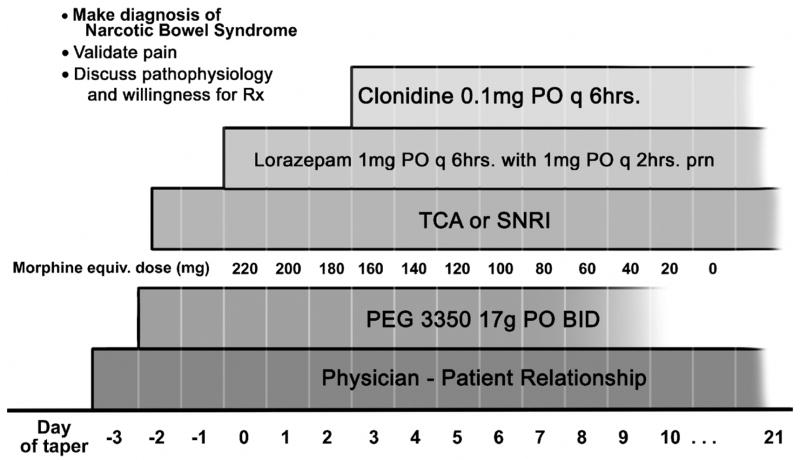
Figure 4.
Narcotic Withdrawal Protocol for NBS. After identification of NBS and discussion with the patient (validating pain, discussion of pathophysiology and willingness to start therapy) the taper starts with a hypothetical dosage of 250 mg/day and then weaned at a 10–33% reduction rate per day. Polyethylene glycol (PEG) is used to treat constipation as needed. A tricyclic or serotonin-norepinephrine (SNRI) antidepressant is started before detoxification and continues indefinitely for pain control. Lorazepam added at the outset of therapy for withdrawal anxiety and is discontinued at the end of the narcotic taper. Clonidine is typically added after day 2 or 3 and is continued until withdrawal is completed or for several weeks later. Of paramount importance is an ongoing physician-patient relationship.
Narcotic Withdrawal Protocol
Patients with nausea and vomiting or intestinal ileus or pseudo-obstruction (e.g. cases #2 and 4) may require inpatient treatment with a nasogastric tube for decompression. Conversely, patients using narcotics more chronically for the abdominal pain can be slowly tapered as outpatients. In situations where the physician perceives limited motivation and social support, an inpatient withdrawal program may provide the needed support (case #5).
The time frame of the withdrawal may be influenced by the duration of the narcotic usage and the dose of narcotics. A patient who develops the syndrome over a short time frame and is using high dose narcotics prescribed post-operatively should be able to tolerate a shorter withdrawal period (case #1 and #2). In contrast, a former intravenous drug user who has subscribed to maintenance methadone therapy with escalating doses may require a longer course (case #4).
When instituting the withdrawal, the patient should initially be covered with the maximal dose that will achieve comfort; usually this is the dose chronically being used or is currently being used in the inpatient setting. Patients taking intravenous dosages should continue with the intravenous route and then switched over to an oral narcotic equivalent in a few days. Patients already on oral agents can continue to be withdrawn on oral agents.
Based on our experience, a general guideline is to decrease the starting dosage by 10–33% per day using a medium to long acting narcotic. The medication should be given in equally divided dosages in a non-contingent fashion rather than as needed to prevent the effect of acute withdrawal and to avoid urgent requests for medication for re-emerging pain. For patients taking shorter acting agents (e.g., oxycodone) conversion to methadone can prevent the effect of acute withdrawal. However, the shorter acting agents can still be used if the medication is given more frequently, e.g., every 3 hours. Conversion tables (e.g. with Epocrates®) should be used to be certain that the dosage with another agent has equal analgesic effects. Using this method, full detoxification can occur in 3 to 10 days.
Concomitant Treatments
Concomitant medications are used either to prevent withdrawal symptoms, to reduce anxiety or treat psychological co-morbidity and to provide long-term central analgesia (Table 4). When a rapid withdrawal is undertaken, usually in the hospital, the patient’s clinical state needs to be evaluated at least every several hours for the first day to watch for orthostatic hypotension and syncope, urinary retention or cardiac arrhythmias. For the first several days it is acceptable to prescribe benzodiazepines which should be reduced after withdrawal is complete.
Table 4.
Treatments for Narcotic Bowel Syndrome
Antidepressants
|
Sympatholytics
|
Anxiolytic
|
Spasmolytic
|
Cathartic
|
Psychological Treatment
|
Future therapies
|
Antidepressant
An antidepressant should be started prior to narcotic withdrawal and continued indefinitely. These drugs improve general well-being and abdominal pain (66) (67;68). However, it is important to help the patient understand that full benefit may not occur for several weeks. Tricyclic antidepressants (TCA) are favored because their noradrenergic action is effective in managing pain (69) independent of its antidepressant effects (70), however the anticholinergic and antihistaminic side effects can lead to constipation and orthostasis. A secondary amine TCA (e.g., desipramine, nortriptyline) has fewer of these side effects and this is preferred over the tertiary amine agents (e.g., amitriptyline, imipramine). Lower dosages (e.g. 50 – 75 mg. desipramine) can be used for analgesic effect unless concomitant major depression is identified, which would require full dosage.. A serotonin – noradrenergic reuptake inhibitors (SNRI – e.g., duloxetine) has the advantage of providing pain benefit via its noradrenergic action, yes does not have the bowel related side effects. Selective serotonin reuptake inhibitors (SSRI). (e.g., paroxetine, fluoxetine, citalopram), are not generally recommended since their benefit in pain management is less established.
Benzodiazepine
We recommend the temporary use of a medium to long acting benzodiazepine (e.g. lorazepam, clonazepam) to reduce the anxiety associated with narcotic withdrawal. Lorazepam 1 mg. q6–8 hours, as an example is begun immediately and given throughout the withdrawal period. Non-contingent dosing is needed during the weaning period to avoid breakthrough symptoms of sympathetic activation. The medication should be tapered off when the narcotic withdrawal is completed.
Clonidine
Clonidine, an alpha-2 adrenergic receptor agonist, is effective in reducing the sympathetic symptoms of narcotic withdrawal including anxiety, restlessness, muscle pains and chills, (71;72). Clonidine has CNS effects by blocking activity of the locus ceruleus, an anxiety center (73) and the peripheral intestinal effects by decreasing acetylcholine release from pre-synaptic terminals thus decreasing the strength, frequency and severity of motor contractions (74). This agent is started toward the end of the taper as the withdrawal symptoms start. A typical starting dose is 0.1mg TID and titrated up to the desired effect (up to 0.6mg/day) while monitoring blood pressure and orthostasis. Particularly for outpatients, a clonidine patch may be applied for steady dosing and improved compliance. This medication can be tapered off after narcotic withdrawal or alternatively continued indefinitely, since clonidine has independent effects on relieving functional GI symptoms including pain and diarrhea (75;76)
Treatment of Constipation
If patients have constipation and/or pain improves after bowel movements and there is no evidence for a bowel obstruction, treatment is warranted. Polyethylene glycol solutions (PEG) act as osmotic laxatives and are a reasonable choice. Other options include tegaserod (77) or Lubiprostone (78). While these agents have been shown to be effective in chronic idiopathic constipation, their efficacy in opioid bowel dysfunction has not been fully studied. Phosphate and magnesium based osmotic preparations or stimulant laxatives should be avoided because of potential electrolyte disturbances. More recently novel opioid peripherally acting agents such as alvimopan which blocks the μ receptor (79) and N-methylnaltrexone are under study and may have benefit for opioid induced constipation and ileus(80) (81) since they preserve the central analgesic effects while blocking the gastrointestinal sites of action.
Psychological Treatments
Psychological treatment is a rational long-term option, though there are no studies yet to confirm our clinical observations. Numerous studies do attest to the value of cognitive behavioral and other therapies in reducing experience of pain symptoms (82). Ongoing psychological treatment with a specialist in behavioral medicine can provide benefits. By teaching patients non pharmacologic techniques to manage their symptoms (distraction, relaxation, and focused attention), patients are helped to achieve a sense of control over their symptoms. Psychologists can also work with patients to reduce negative thinking and problem solving that may contribute to an increase in perception of symptoms. Finally, psychologists often work with patients to develop strategies to address effects of their NBS on relationships, employment and associated difficulties.
Some patients may become apprehensive or feel threatened by a recommendation to work with a psychologist. It should be emphasized to the patient that this recommendation does not imply symptoms of mental illness or narcotic addiction. Rather, it is a component of a comprehensive approach to the management of their condition and is designed to help reduce their overall negative symptom experience.
Our treatment program is shown in Figure 4, which assumes a daily narcotic usage of 250 mg. of morphine equivalent. Prior to the taper of narcotics, a diagnosis must be made and the physician-patient relationship established. Once the patient agrees to the treatment program, detoxification begins and the treatments instituted according to the schedule shown. The treating physician needs to continue with the care of the patient after the detoxification has occurred.
Issues That May Interfere with a Successful Outcome
Some patients may “negotiate” on the treatment protocol after it has started, i.e., to request stopping the narcotic taper for a day or two, or to request more narcotics for a “flare”. Here the physician must determine if this relates to: anxiety about a possible treatment failure by someone motivated to continue the treatment, ambivalence or a lack of desire to continue, or in rare cases, malingering behavior. In all cases, the physician needs to explore and discuss the patient’s concerns in more detail. The former situation may occur when insufficient attention was paid to the patient’s concerns before initiating the program and perhaps by not initially having the patient agree to the protocol. An explanation about the value of continuing the treatment and the fact that the patient won’t be left alone in pain may help. It is possible to make some modifications (e.g., to increase the benzodiazepine, reduce the time between narcotic dosing without increasing the total dose, consider a non-narcotic analgesic such as Ketorolac (83), or merely to agree to come back for another discussion) that may eliminate the problem. If the patient seems unwilling to engage in an interactive discussion and only requests narcotics, then the patient is probably not motivated to continue. As with case #5, this may indicate drug-seeking behavior, and it is best to stop the program.
Resistance toward undertaking an unfamiliar or untested treatment strategy may occur, especially when the narcotic medication has provided some measurable benefit to the patient at some point. Here, time must be taken to discuss the benefits and risks of the previous course of treatment before discussing the value of a change. When patients are given an opportunity to openly discuss the positives and negatives in this manner, they become more aware of the limitations of the prior treatment, and increases motivation to try something new. (84)
Not infrequently, when detoxification is attempted on an outpatient basis as with Case #3, the patient may paradoxically taper the narcotic too rapidly or abruptly discontinue it and phone in distress while experiencing withdrawal symptoms. Here, the patient may not have understood the withdrawal protocol, was trying to “prove” he or she can do it and wants to get it over with, or rather was actually unwilling to be on the program and “sabotages” it through this behavior (“See, it doesn’t work”). Again efforts must be made to explore with the patient the reasons for this occurrence and then by using that information the physician may proceed to reinstitute the protocol, hopefully with greater patient motivation.
Patients with NBS may see many physicians for treatment: to find the right physician who can provide a “cure,” because of a lack of trust of the physicians’ diagnoses, or out of frustration with demeaning or critical attitudes communicated by other physicians. They may leave the hospital or the office of the treating physician and go to another physician who may again prescribe narcotics, especially if he or she is unfamiliar with the withdrawal treatment plan. Ideally, the treating physician needs to have the patient agree to work with one treating physician, to identify all the other physicians involved and then send a copy of the clinic note or discharge summary to them to increase the chance for success with the treatment program. Any outpatient withdrawal treatment program is confounded by the fact that medical culture has given license to emergency departments to inadvertently use intravenous narcotics as a means to quickly treat and discharge the patient with the expectation that the primary care or referring physician will follow-up. Occasionally the patient may actually be seeing another physician or presenting to emergency departments for narcotics while allegedly continuing with the withdrawal program. This behavior is a poor prognostic sign, and supports drug seeking behavior.
Conclusion
In the United States, narcotics are now one of the most commonly prescribed medications for pain, and their use is growing. Furthermore, there has been a shift from prescribing narcotics for acute or malignant pain to chronic non-malignant pain including the FGIDs where patients are more vulnerable to the development of the narcotic bowel syndrome. NBS occurs when patients have an increased or unresponsive pain experience along with a variety of GI motility disturbances related to the narcotics. The diagnosis is based on positive criteria and can easily be identified. However several factors in combination can perpetuate the failure to recognize NBS and potentially increase its prevalence in patients with chronic pain. Such factors include when there is uncertainty in the diagnosis among the patients who exhibit pain behaviors (“furor medicus”)(24), when a diagnosis is present that “justifies” the use of narcotics, even when the disease is not active or in rare cases among patients who report severe pain as a means to obtain narcotics. Furthermore, the health care system can amplify this effect by its focus on rapid “throughput” and profit margins via diagnostics and procedures to exclude other diseases rather than by using cognitive skills to understand the patient and problem.
In this paper we describe the diagnostic and clinical features of NBS and an approach to treatment. We are hopeful that early recognition of the syndrome coupled with a recommended method to treat the disorder will lead to better patient satisfaction, greater attention to the physician’s and health care system’s behaviors relating to pain and narcotics and ultimately to improved clinical outcomes. The cornerstone of care will depend on an effective physician-patient relationship.
Table 2.
Case Summaries
| Age/Gender | Symptoms (Duration) | Duration Of Narcotic Use | Dose At Presentation | Treatment For NBS | Immediate Outcome | Follow Up |
|---|---|---|---|---|---|---|
| #1 40 yo Female | No prior GI symptoms or narcotic use had surgery for acute pain | 10 weeks during post-operative period while in hospital | 40 mg/day morphine sulphate IV escalating to 80 mg/day | Narcotic withdrawal over 6 days, clonidine0.1 po tid, lorazepam01 mg tid, desipramine50 mg. qhs | 75% relief by hospital discharge | Complete resolution at 1 year and no recurrence |
| #2 37 yo Female | abdominal pain, nausea, vomiting, diarrhea, constipation.(14 years) | 2 years | Morphine Sulfate90mg/day | Narcotic reduced 33% q2days, clonidine patch 0.2 mg before stopping narcotic, desipramine 100 mg qhs, paroxetine 20 mg qd | Marked improvement | 3 months: no pain; on desipramine and paroxetine with mild constipation 9 months: 3 mild episodes of pain consistent with IBS; No narcotic use |
| #3 42 yo Female | IBS (23 years) + chronic pain (3 years) | 3 years | Oxycodone 30mg/day | Narcotic reduced 50% per week, Clonazepam 0.5 mg qd, clonidine patch 0.2 mg, paroxetine 60 mg. qd | Marked improvement | One year later, no pain. No narcotics. |
| #4 20 yo Female | Lower Back Pain (16 months) abdominal pain, nausea, vomiting (4 weeks); New diagnosis of CD | 20 months | Methadone260mg/day | Narcotic reduced 10–20% over 11 days, Duloxetine 30–60 mg, cyclobenzaprine, clonidine 0.1 po qid, lorazepam 1 mg. po qid | Marked improvement relapse and subsequent improvement, | 6 months, no pain or narcotics; 12 months relapse of narcotics, again detoxified and pain free one month later |
| #5 20 yo Female | Mid-abdominal pain 8 weeks (history of UC with IPAA 1 year earlier) | At least 8 weeks (uncertain if longer | 90 mg. IV increased to 360 mg. morphine or equivalent/day | Narcotic reduction by 33% every two days, Duloxetine, clonidine, lorazepam | Narcotics withdrawn but patient went back on narcotics | Continued drug seeking behavior several months later |
Acknowledgments
This review was supported by the Gastrointestinal Biopsychosocial Research Center at the University of North Carolina (NIH R24 DK067674), and the UNC Center for Functional GI and Motility Disorders. We would like to thank the GI fellows and physician assistants of the Division of Gastroenterology and Hepatology for their assistance in the care of these patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182:11S–8S. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- 2.Mehendale SR, Yuan CS. Opioid-induced gastrointestinal dysfunction. Dig Dis. 2006;24:105–12. doi: 10.1159/000090314. [DOI] [PubMed] [Google Scholar]
- 3.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–71. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 4.Sandgren JE, McPhee MS, Greenberger NJ. Narcotic bowel syndrome treated with Clonidine. Ann Intern Med. 1984;101:331–34. doi: 10.7326/0003-4819-101-3-331. [DOI] [PubMed] [Google Scholar]
- 5.Rogers M, Cerda JJ. Editorial: The narcotic bowel syndrome. J Clin Gastroenterol. 1989;11:132–35. [PubMed] [Google Scholar]
- 6.Wong V, Sobala G, Losowsky M. A case of narcotic bowel syndrome successfully treated with clonidine. Postgrad Med J. 1994;70:138–40. doi: 10.1136/pgmj.70.820.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Qu Q, Chen Y. Heroin addiction and surgical abdominal pain: report of three cases. Chinese Journal of Internal Medicine. 1995;34:46–48. [PubMed] [Google Scholar]
- 8.Spiller R, Campbell E. Post-infectious irritable bowel syndrome. Curr Opin Gastroenterol. 2006;22:13–17. doi: 10.1097/01.mog.0000194792.36466.5c. [DOI] [PubMed] [Google Scholar]
- 9.Drossman DA. The Functional Gastrointestinal Disorders and the Rome III Process. In: Drossman DA, Corazziari E, Delvaux M, Spiller R, Talley N, Thompson WG, et al., editors. Rome III: The Functional Gastrointestinal Disorders. 3. McLean, VA: Degnon Associates, Inc; 2006. [Google Scholar]
- 10.Longstreth GF, Drossman DA. Severe Irritable Bowel And Functional Abdominal Pain Syndromes: Managing The Patient And Health Care Costs. Clinical Gastroenterology and Hepatology. 2005;3:397–400. doi: 10.1016/s1542-3565(05)00084-4. [DOI] [PubMed] [Google Scholar]
- 11.Drossman DA, Li Z, Leserman J, Keefe FJ, Hu YJ, Toomey TC. Effects of coping on health outcome among female patients with gastrointestinal disorders. Psychosom Med. 2000;62:309–17. doi: 10.1097/00006842-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Drossman DA. Psychosocial Sound Bites: Exercises in the patient-doctor relationship. Am J Gastroenterol. 1997;92:1418–23. [PubMed] [Google Scholar]
- 13.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA Technical Review on Irritable Bowel Syndrome. Gastroenterol. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 14.Drossman DA. Functional abdominal pain syndrome. Clinical Gastroenterology and Hepatology. 2004;2:353–65. doi: 10.1016/s1542-3565(04)00118-1. [DOI] [PubMed] [Google Scholar]
- 15.Drossman DA. The functional gastrointestinal disorders, their diagnosis, and the Rome II process. In: Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE, Rome II, editors. The functional gastrointestinal disorders: Diagnosis, pathophysiology and treatment; A multinational consensus. 2. 2000. pp. 1–29. [Google Scholar]
- 16.Lembo A, Ameen V, Drossman DA. Irritable bowel syndrome: toward an understanding of severity. Clinical Gastroenterology and Hepatology. 2005;3:717–25. doi: 10.1016/s1542-3565(05)00157-6. [DOI] [PubMed] [Google Scholar]
- 17.Gray DW, Collin J. Non-specific abdominal pain as a cause of acute admission to hospital. Br J Surg. 1987;74:239–42. doi: 10.1002/bjs.1800740404. [DOI] [PubMed] [Google Scholar]
- 18.Drossman DA. Brain Imaging and its Implications for Studying Centrally Targeted Treatments in IBS: A Primer for Gastroenterologists. Gut. 2005;54:569–73. doi: 10.1136/gut.2004.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clouse RE, Mayer EA, Aziz Q, Drossman DA, Dumitrascu DL, Monnikes H, et al. Functional Abdominal Pain Syndrome. In: Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, et al., editors. Rome III: The Functional Gastrointestinal Disorders. 3. McLean, VA: Degnon Associates, Inc; 2006. [Google Scholar]
- 20.Clouse RE, Mayer EA, Aziz Q, Drossman DA, Dumitrascu DL, Monnikes H, et al. Functional Abdominal Pain Syndrome. Gastroenterol. 2006;130:1492–97. doi: 10.1053/j.gastro.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 21.Longstreth GF, Yao JF. Irritable bowel syndrome and surgery: A multivariable analysis. Gastroenterol. 2004;126:1665–73. doi: 10.1053/j.gastro.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Turk DC, Okifuji A. What factors affect physicians’ decisions to prescribe opioids for chronic noncancer pain patients? Clinical Journal of Pain. 1997;13:330–336. doi: 10.1097/00002508-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Drossman DA. Medicine has become a business. But what is the cost? Gastroenterol. 2004;126:952–53. doi: 10.1053/j.gastro.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 24.DeVaul RA, Faillace LA. Persistent pain and illness insistence - A medical profile of proneness to surgery. Am J Surg. 1978;135:828–33. doi: 10.1016/0002-9610(78)90176-9. [DOI] [PubMed] [Google Scholar]
- 25.Trescot AM, Boswell MV, Atluri SL, Hansen HC, Deer TR, Abdi S, et al. Opioid guidelines in the management of chronic non-cancer pain. Pain Physician. 2006;9:1–39. [PubMed] [Google Scholar]
- 26.Sandoval JA, Furlan AD, Mailis-Gagnon A. Oral methadone for chronic noncancer pain: a systematic literature review of reasons for administration, prescription patterns, effectiveness, and side effects. Clin J Pain. 2005;21:503–12. doi: 10.1097/01.ajp.0000146165.15529.50. [DOI] [PubMed] [Google Scholar]
- 27.Watkins A, Wasmann S, Dodson L, Hayes M. An evaluation of the care provided to patients prescribed controlled substances for chronic nonmalignant pain at an academic family medicine center. Fam Med. 2004;36:487–89. [PubMed] [Google Scholar]
- 28.France RD, Urban BJ, Keefe FJ. Long-term use of narcotic analgesics in chronic pain. Soc Sci Med. 1984;19:1379–82. doi: 10.1016/0277-9536(84)90027-3. [DOI] [PubMed] [Google Scholar]
- 29.Fordyce WE, Fowler RS, Delateur B. Case histories and shorter communications: An application of behavior modification technique to a problem of chronic pain. Behavior Research and Therapy. 1968;6:105–7. doi: 10.1016/0005-7967(68)90048-x. [DOI] [PubMed] [Google Scholar]
- 30.Ackerman SJ, Mordin M, Reblando J, Xu X, Schein J, Vallow S, et al. Patient-reported utilization patterns of fentanyl transdermal system and oxycodone hydrochloride controlled-release among patients with chronic nonmalignant pain. J Manag Care Pharm. 2003;9:223–31. doi: 10.18553/jmcp.2003.9.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcus DA. Treatment of nonmalignant chronic pain. Am Fam Physician. 2000;61:1331–36. [PubMed] [Google Scholar]
- 32.Moulin DE, Iezzi A, Amireh R, Sharpe WK, Boyd D, Merskey H. Randomised trial of oral morphine for chronic non-cancer pain. Lancet. 1996;347:143–47. doi: 10.1016/s0140-6736(96)90339-6. [DOI] [PubMed] [Google Scholar]
- 33.McGarrity TJ, Peters DJ, Thompson C, McGarrity SJ. Outcome of patients with chronic abdominal pain referred to chronic pain clinic. The American Journal of Gastroenterology. 2000;95:1812–16. doi: 10.1111/j.1572-0241.2000.02170.x. [DOI] [PubMed] [Google Scholar]
- 34.Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 2):17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 35.Crain SM, Shen KF. Opioids can evoke direct receptor-mediated excitatory effects on sensory neurons. TiPS. 1990;11:77–81. doi: 10.1016/0165-6147(90)90322-y. [DOI] [PubMed] [Google Scholar]
- 36.Crain SM, Shen KF. Antagonists of excitatory opioid receptor functions enhance morphine’s analgesic potency and attenuate opioid tolerance/dependence liability. Pain. 2000;84:121–31. doi: 10.1016/s0304-3959(99)00223-7. [DOI] [PubMed] [Google Scholar]
- 37.Crain SM, Shen KF. After chronic opioid exposure sensory neurons become supersensitive to the excitatory effects of opioid agonists and antagonists as occurs after acute elevation of GM1 ganglioside. Brain Res. 1992;575:13–24. doi: 10.1016/0006-8993(92)90417-8. [DOI] [PubMed] [Google Scholar]
- 38.Amass L, Ling W, Freese TE, Reiber C, Annon JJ, Cohen AJ, et al. Bringing buprenorphine-naloxone detoxification to community treatment providers: the NIDA Clinical Trials Network field experience. Am J Addict. 2004;13(Suppl 1):S42–S66. doi: 10.1080/10550490490440807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ossipov MH, Lai J, King T, Vanderah TW, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers. 2005;80:319–24. doi: 10.1002/bip.20254. [DOI] [PubMed] [Google Scholar]
- 40.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–25. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 41.Fields HL. Is there a facilitating component to central pain modulation? American Pain Society Journal. 1992;1:71–78. [Google Scholar]
- 42.King T, Ossipov MH, Vanderah TW, Porreca F, Lai J. Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals. 2005;14:194–205. doi: 10.1159/000087658. [DOI] [PubMed] [Google Scholar]
- 43.Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, et al. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 2000;20:7074–79. doi: 10.1523/JNEUROSCI.20-18-07074.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bian D, Ossipov MH, Ibrahim M, Raffa RB, Tallarida RJ, Malan TP, Jr, et al. Loss of antiallodynic and antinociceptive spinal/supraspinal morphine synergy in nerve-injured rats: restoration by MK-801 or dynorphin antiserum. Brain Res. 1999;831:55–63. doi: 10.1016/s0006-8993(99)01393-1. [DOI] [PubMed] [Google Scholar]
- 45.Larcher A, Laulin JP, Celerier E, Le MM, Simonnet G. Acute tolerance associated with a single opiate administration: involvement of N-methyl-D-aspartate-dependent pain facilitatory systems. Neuroscience. 1998;84:583–89. doi: 10.1016/s0306-4522(97)00556-3. [DOI] [PubMed] [Google Scholar]
- 46.Ghilardi JR, Allen CJ, Vigna SR, McVey DC, Mantyh PW. Trigeminal and dorsal root ganglion neurons express CCK receptor binding sites in the rat, rabbit, and monkey: possible site of opiate-CCK analgesic interactions. J Neurosci. 1992;12:4854–66. doi: 10.1523/JNEUROSCI.12-12-04854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinricher MM, McGaraughty S, Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventromedial medulla. J Neurophysiol. 2001;85:280–286. doi: 10.1152/jn.2001.85.1.280. [DOI] [PubMed] [Google Scholar]
- 48.Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004;92:1982–89. doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan H, Fields HL. Hyperalgesia during acute opioid abstinence: evidence for a nociceptive facilitating function of the rostral ventromedial medulla. J Neurosci. 1991;11:1433–39. doi: 10.1523/JNEUROSCI.11-05-01433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 51.Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–69. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Chapman GA, Moores K, Harrison D, Campbell CA, Stewart BR, Strijbos PJ. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J Neurosci. 2000;20:RC87. doi: 10.1523/JNEUROSCI.20-15-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–65. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saab CY, Wang J, Gu C, Garner KN, Al-Chaer ED. Microglia: A newly discovered role in visceral hypersensitivity. Neuron Glia Biology. 2007:1–7. doi: 10.1017/S1740925X07000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rattan AK, Tejwani GA. Effect of chronic treatment with morphine, midazolam and both together on dynorphin(1-13) levels in the rat. Brain Res. 1997;754:239–44. doi: 10.1016/s0006-8993(97)00084-x. [DOI] [PubMed] [Google Scholar]
- 56.Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–86. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 57.Nichols ML, Lopez Y, Ossipov MH, Bian D, Porreca F. Enhancement of the antiallodynic and antinociceptive efficacy of spinal morphine by antisera to dynorphin A (1–13) or MK-801 in a nerve-ligation model of peripheral neuropathy. Pain. 1997;69:317–22. doi: 10.1016/S0304-3959(96)03282-4. [DOI] [PubMed] [Google Scholar]
- 58.Chang L, Drossman DA. Optimizing patient care: The psychosocial interview in the irritable bowel syndrome. Clinical perspectives in gastroenterology. 2002;5:336–41. [Google Scholar]
- 59.Drossman DA. The Physician-Patient Relationship. In: Corazziari E, editor. Approach to the Patient with Chronic Gastrointestinal Disorders. Milan: Messaggi; 1999. pp. 133–39. [Google Scholar]
- 60.Drossman DA, Chang L. Psychosocial factors in the care of patients with GI disorders. In: Yamada T, editor. Textbook of Gastroenterology. Philadelphia: Lippincott-Raven; 2003. pp. 636–54. [Google Scholar]
- 61.Drossman DA. Diagnosing and treating patients with refractory functional gastrointestinal disorders. Ann Intern Med. 1995;123:688–97. doi: 10.7326/0003-4819-123-9-199511010-00008. [DOI] [PubMed] [Google Scholar]
- 62.Stewart M, Brown JB, Boon H, Galajda J, Meredith L, Sangster M. Evidence on patient-doctor communication. Cancer Prev Control. 1999;3:25–30. [PubMed] [Google Scholar]
- 63.Clouse RE, Mayer EA, Aziz Q, Drossman DA, Dumitrascu DL, Monnikes H, et al. Functional Abdominal Pain. Gastroenterol. 2005 doi: 10.1053/j.gastro.2005.11.062. In press. [DOI] [PubMed] [Google Scholar]
- 64.Kosten TR, O’Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348:1786–95. doi: 10.1056/NEJMra020617. [DOI] [PubMed] [Google Scholar]
- 65.Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949–58. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 66.Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, et al. Cognitive-Behavioral Therapy vs. education and Desipramine vs. Placebo for Moderate to Severe Functional Bowel Disorders. Gastroenterol. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 67.Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with anti- depressants: A meta-analysis. Am J Med. 2000;108:65–72. doi: 10.1016/s0002-9343(99)00299-5. [DOI] [PubMed] [Google Scholar]
- 68.Thiwan SM, Drossman DA. Treatment of functional GI disorders with psychotropic medicines: A review of evidence with a practical approach. Gastroenterology & Hepatology. 2006;2:678–88. [PMC free article] [PubMed] [Google Scholar]
- 69.Maizels M, McCarberg B. Antidepressants and antiepileptic drugs for chronic non-cancer pain. Am Fam Physician. 2005;71:483–90. [PubMed] [Google Scholar]
- 70.Brannan SK, Mallinckrodt CH, Brown EB, Wohlreich MM, Watkin JG, Schatzberg AF. Duloxetine 60 mg once-daily in the treatment of painful physical symptoms in patients with major depressive disorder. J Psychiatr Res. 2005;39:43–53. doi: 10.1016/j.jpsychires.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 71.Gossop M. Clonidine and the treatment of the opiate withdrawal syndrome. Drug Alcohol Depend. 1988;21:253–59. doi: 10.1016/0376-8716(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 72.O’Connor P, Carroll KM, Shi JM, Schottenfeld RS, Kosten TR, Rounsaville BJ. Three methods of opioid detoxification in a primary care setting. Ann Intern Med. 1997;127:526–30. doi: 10.7326/0003-4819-127-7-199710010-00004. [DOI] [PubMed] [Google Scholar]
- 73.Gold MS, Pottash AC. The neurobiological implication of clonidine HCl. Ann N Y Acad Sci. 1981;362:191–202. doi: 10.1111/j.1749-6632.1981.tb12809.x. [DOI] [PubMed] [Google Scholar]
- 74.Seiler R, Rickenbacher A, Shaw S, Balsiger BM. alpha- and beta-adrenergic receptor mechanisms in spontaneous contractile activity of rat ileal longitudinal smooth muscle. J Gastrointest Surg. 2005;9:227–35. doi: 10.1016/j.gassur.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 75.Camilleri M, Kim DY, McKinzie S, Kim HJ, Thomforde G, Burton D, et al. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 2003;1:111–21. doi: 10.1053/cgh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 76.Viramontes B, Malcolm A, Szarka L, McKinzie S, Burton D, Kost L, Zinsmeister A, Camilleri M. Dose-related effects of α2-adrenergic agent, clonidine, on human gastrointestinal motor, transit and sensory functions. Gastroenterology. 2000;118:A666. Ref Type: Abstract. [Google Scholar]
- 77.Quigley EM, Wald A, Fidelholtz J, Boivin M, Pecher E, Earnest D. Safety and tolerability of tegaserod in patients with chronic constipation: pooled data from two phase III studies. Clin Gastroenterol Hepatol. 2006;4:605–13. doi: 10.1016/j.cgh.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 78.Johanson J, Panas R, Holland PC, Ueno R. A dose-ranging, double-blind, placebo-controlled study of lubiprostone in patients with irritable bowel syndrome with constipation. Neurogastroenterology & Motility. 2006;18(8):730. Ref Type: Abstract. [Google Scholar]
- 79.Paulson DM, Kennedy DT, Donovick RA, Carpenter RL, Cherubini M, Techner L, et al. Alvimopan: an oral, peripherally acting, mu-opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction--a 21-day treatment-randomized clinical trial. J Pain. 2005;6:184–92. doi: 10.1016/j.jpain.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Camilleri M. Alvimopan, a selective peripherally acting mu-opioid antagonist. Neurogastroenterol Motil. 2005;17:157–65. doi: 10.1111/j.1365-2982.2005.00640.x. [DOI] [PubMed] [Google Scholar]
- 81.Lembo A. Peripheral opioids for functional GI disease: a reappraisal. Dig Dis. 2006;24:91–98. doi: 10.1159/000090312. [DOI] [PubMed] [Google Scholar]
- 82.Lackner JM, Mesmer C, Morley S, Dowzer C, Hamilton S. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. Journal of Consulting & Clinical Psychology. 2004;72:1100–1113. doi: 10.1037/0022-006X.72.6.1100. [DOI] [PubMed] [Google Scholar]
- 83.Joishy SK, Walsh D. The opioid-sparing effects of intravenous ketorolac as an adjuvant analgesic in cancer pain: application in bone metastases and the opioid bowel syndrome. J Pain Symptom Manage. 1998;16:334–39. doi: 10.1016/s0885-3924(98)00081-5. [DOI] [PubMed] [Google Scholar]
- 84.DiClemente CC, Prochaska JO, Gibertini M. Self-efficacy and the stages of self-change of smoking. Cognitive Therapy and Research. 2007;9:181–200. [Google Scholar]