Abstract
The therapy of focal epilepsy remains unsatisfactory for as many as 25% of patients. We tested the hypothesis that an efficient, ultraviolet light emitting diode (UV LED), coupled with a newly developed “caged” γ-aminobutyric acid (GABA), might be capable of terminating “ictal-like” events in cultured murine neurons. GABA was released from BC204, a recently described caged GABA, using a small, ultraviolet (UV) LED. Ictal-like events were provoked by removal of extracellular magnesium. In preliminary control experiments, the concentration of GABA released from our caged compound was dependent upon the strength and duration of the illumination, and readily achieved micromolar (μM) levels that are known to activate tonic, extrasynaptic GABAA receptors. Ultraviolet illumination had no effect when BC204 was not present in the perfusate and the currents produced by BC204 were eliminated by picrotoxin. Within a few seconds of UV illumination, BC204 rapidly terminated ictal-like events at low μM concentration. Uncaging of BC204 also blocked the elevation of intracellular calcium induced by seizure like discharges in our cultures. While much more technical development is clearly required to extend our observations to a more intact preparation, these results suggest the intriguing possibility of constructing an implantable device to “optically suppress” focal human seizures under closed loop control.
Keywords: caged compounds, diode, epilepsy, GABA, hippocampus, LED
1. Introduction
The therapy of epilepsy remains inadequate for at least a quarter of those affected. so that many patients continue to experience seizures and side effects from antiepileptic medications. Surgical resection is an option for some patients whose seizures arise from a neocortical or temporal lobe “focus”. While the latter cohort has a high probability of responding to surgery, the success rate in the former group is typically below 60%. Even in those patients who benefit from surgery, there is concern about neuropsychological morbidity from the removal of functioning brain tissue (Alpherts et al., 2006;Rausch et al., 2003).
In order to avoid this morbidity, several alternatives to irreversible resection have been proposed as possible treatments for focal epilepsy, including focal brain stimulation, local drug delivery, and cooling (Eder et al., 1997;Theodore and Fisher, 2004;Yang and Rothman, 2001). In an attempt to exploit the exquisite spatial and temporal resolution of optical activation, we recently began developing a different technology to terminate focal seizures. A small, powerful ultraviolet (UV) light emitting diode (LED) is employed to release γ-aminobutyric acid (GABA) from a recently developed “caged GABA” analog, 4-[[(2H-benzopyran-2-one-7-amino-4-methoxy)carbonyl]amino]butanoic acid (BC204)(Curten et al., 2005). This generates low micromolar (μM) concentrations of GABA without the use of lasers or high power lamps. This GABA release is sufficient to activate GABAA receptors (likely tonic) and limit paroxysmal neuronal firing in cultured central neurons (Farrant and Nusser, 2005;Scimemi et al., 2005). The results described here indicate that it may be possible to couple a miniature, implantable UV LED to a reservoir that releases caged GABA close to epileptogenic neocortex. Closed loop activation of the LED could quickly elevate ambient GABA levels and suppress seizures, without globally depressing central nervous system functioning.
2. Methods
2.1 Tissue culture
Hippocampal neurons were obtained from 16 day embryonic Swiss Webster mice and grown on polylysine-coated 15 mm glass cover slips (Assistant; Sondheim, Germany), using methods previously described in great detail (Thio and Zhang, 2006). Two cover slips were placed on the bottom of a plastic 35 mm Petri dish and approximately 500,000 dissociated cells in Minimal Essential Media supplemented with 10% Nu-Serum (BD Biosciences; Bedford, MA)were placed in each dish. For the calcium imaging experiments, neocortical cultures that had been plated on a single glass coverslip glued to the bottom of a 35 mm plastic dish were used (MatTek; Ashland, MA).
2.2 Physiology
Cultures between 10 and 21 days in vitro were used for intracellular recording. The glass coverslips were transferred to an aluminum dish with a glass bottom that held about 0.5 ml and was mounted on the stage of an inverted microscope. In the initial experiments that characterized BC204 uncaging and developed the GABA and BC204 concentration response curves, the cultures were perfused with a balanced salt solution containing (mM): NaCl 140; KCl 3.0; HEPES 10; CaCl2 1.0; MgSO4 4.0; and glucose 5.5 (pH 7.4). Recording pipettes were fabricated from thin-walled capillary tubing (BF120-90; Friedrich & Dimmock, Millville, NJ) using a vertical puller (700D; David Kopf Instruments, Tujunga, CA). Pipette resistances were 4 – 8 MΩ when filled with an intracellular solution containing (mM): KCl 140; HEPES 10: glucose 5.5; EGTA 1.1; NaOH 2.2; MgATP 4; and QX314 1. GABA and BC204 were added to the balanced salt solution and applied by whole bath perfusion at approximately 3 ml per minute from gravity driven reservoirs that were manually gated by solenoids (Lee Company, Westbrook, CT).
For experiments in which seizure-like activity was elicited, the stage was warmed to 30° C with a resistance heater. The divalent cation concentrations in the control extracellular perfusate were changed to (mM): CaCl2 2.0 and MgSO4 1.0 and the latter was removed to elicit seizure-like activity. For current clamp recording, pipettes were fabricated from thicker walled capillaries (BF120-68; Friedrich & Dimmock)and had resistances between 10 and 20 MΩ when filled with (mM): potassium gluconate 125; KCl 10; HEPES 10; glucose 5.5; EGTA 1.1; NaOH 2.2; and MgATP 4.
Voltage and current clamp experiments were performed using a patch clamp amplifier (8900; Dagan, Minneapolis, MN) interfaced to a computer (USB-6009; National Instruments, Austin, TX). Amplifier output was filtered prior to digitization and storage. Commercial software was used for data acquisition (VI Logger; National Instruments) and data were converted into text files for offline analysis (pClamp 10; Molecular Devices, Sunnyvale, CA). For the GABA and BC204 concentration response experiments, a slow digitization rate of 10 Hz was used; for capturing the seizure-like events, the acquisition rate was increased to 5 KHz. In both cases the resolution of the A/D converter was 14 bits.
BC204 was uncaged with a high power UV LED (maximum radiant output 100 mW; NCCUO33; Nichia, Tokyo) controlled by a custom built constant current power supply that could be activated by a standard TTL pulse. Detailed specifications for the LED are available (www.nichia.com). The spectral peak of the LED was 365 nm. A metal enclosure and heat sink protected the LED, which was coupled to the culture dish by a fiber optic bundle (2 mm diameter x 16 cm length; CeramOptec; East Longmeadow, MA) capable of passing UV light. The bundle terminated 2–3 mm from the surface of the perfusate and illuminated an oval region approximately 14 x 10 mm that included the recording pipette on the dish bottom. No attempt was made to precisely focus the emitted light. In one set of experiments, we used a lower power UV LED with the same spectral peak (maximum output 2 mW and maximum current 25 mA; NSHU550B; Nichia). The reservoir and tubing containing BC204 were protected from light, and room and microscope lights were turned off whenever BC204 was used.
2.3 Calcium Imaging
We loaded neocortical cultures with fluo-3 AM ester (10 μM; Teflabs, Austin, TX) in 0.2% Pluronic F-127 (Molecular Probes, Eugene, OR) for 30 min at room temperature. After washing with balanced salt solution, they sat for another 30 min to allow for the ester hydrolysis. The cultures were imaged on an inverted microscope (Diaphot Eclipse TE3000, Nikon, Melville, NY) using a 40x, 1.3 N.A fluorite oil immersion objective (Nikon) and a cooled CCD camera (Cooke Corp., Auburn Hill, MI)) equipped with a 75 W xenon arc lamp. The fluorescence excitation was provided by a band-specific filter (485/20; Omega Optical, Brattleboro, VA) in combination with XF73 dichroic beam splitter (Omega Optical, Brattleboro, VA). We collected images at ~2.5 frames per second. After background subtraction, the indicator fluorescence was determined in individual cells and plotted as normalized values reflecting the relative change (F−Fo)/Fo, where F is the indicator intensity at each time point and Fo is the average fluorescence intensity at baseline. Image acquisition and processing were controlled by commercial software (MetaFluor; Molecular Devices, Sunnyvale, CA).
2.4 Statistics
One way repeated measures analysis of variance (or Friedman repeated measures analysis of variance on ranks for non parametric data), followed by the Student-Newman-Keuls test was used for multiple comparisons. When a neuron was exposed to two treatments, a paired t-test was used to determine statistical significance.
3. Results
3.1 UV LED uncages BC204
The original paper that characterized BC204 guided the light from a mercury lamp through a 50 μm quartz fiber and controlled the duration of light exposure with a shutter opened for up to 40 msec (Curten et al., 2005). Initial experiments here were aimed at determining whether our unfocused UV LED would provide sufficient radiant power to uncage a detectable level of GABA from BC204. Neurons (n=4) were voltage clamped at −60 mV in the presence of 10 μM BC204 and illuminated with the power supply set to 200 mA for durations ranging between 1 and 15 seconds. This level of illumination, applied for as little as 1 sec, led to a readily detectable current (Figure 1A). When the exposure was lengthened, the amplitude of the current increased, peaking at 6 sec and slightly decreasing when prolonged to 15 sec, possibly due to desensitization. A 4 sec illumination generated a near maximal current and was used in most of the subsequent experiments (Figure 1B).
Figure 1.
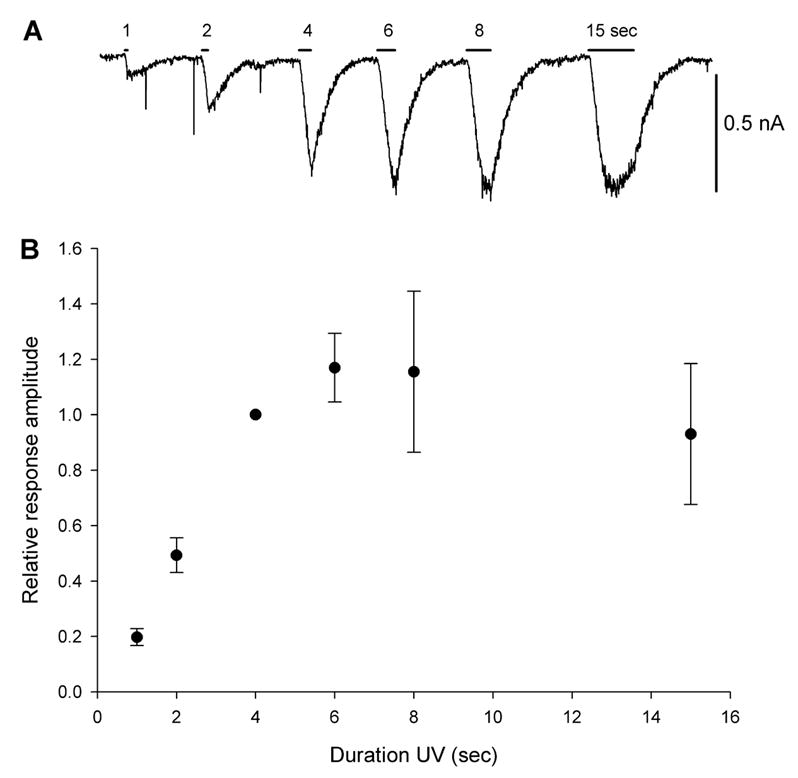
Duration response relationship for BC204 activation with a UV LED. A. Increasing the duration of illumination from 1 to 6 sec lengthens the duration and magnitude of inward current. B. Average peak ± 1 SD current responses for illumination durations up to 15 sec, showing that beyond 6 sec, the response reaches a plateau (n= 4 cells). Values were normalized to 4 sec light application.
Having established that the UV LED could activate detectable currents in the presence of BC204, subsequent experiments examined GABA-mediated concentration response relations. We bath applied GABA at 1, 4, 7 and 10 μM, while recording from neurons in the whole cell configuration (n = 4 – 7 for different GABA concentrations. Currents at the lower three concentrations were normalized to the 10 μM response to obtain a GABA response curve for low concentrations of GABA, the range of interest for these experiments (Figure 2A). We then bath applied BC204, first at 30 μM, and in a subsequent set of experiments at 10 μM, and measured the inward currents produced by 4 sec illumination with decrementing UV LED power (Figure 2B). The inward currents at each level of illumination and BC204 concentration were normalized to the current produced in the same cell by bath perfusion of GABA (10 μM; n = 4 – 13 cells for each current and BC204 concentration; Figure 2B – D). There was a clear relationship between strength of illumination and the magnitude of the current elicited by uncaging BC204. When the BC204 concentration was 30 μM, LED currents ≥ 150 mA gave a response larger than the response produced by bath perfusion of 10 μM GABA, suggesting that a 4 sec light application was capable of uncaging more than one third of the BC204 in the vicinity of the recorded neuron (Figures 2B and C). Even at the highest LED current level, no response was seen in any neuron in the absence of BC204 (not shown). Combining the GABA and BC204 concentration responses allowed us to determine the approximate GABA concentrations produced by different levels of LED illumination (Figure 2D). In the presence of 30 μM BC204, a 25 mA LED current evoked neuronal currents corresponding to about 4 μM GABA. As expected, the illumination intensity had to be increased substantially to produce an equivalent response in the presence of 10 μM BC204.
Figure 2.
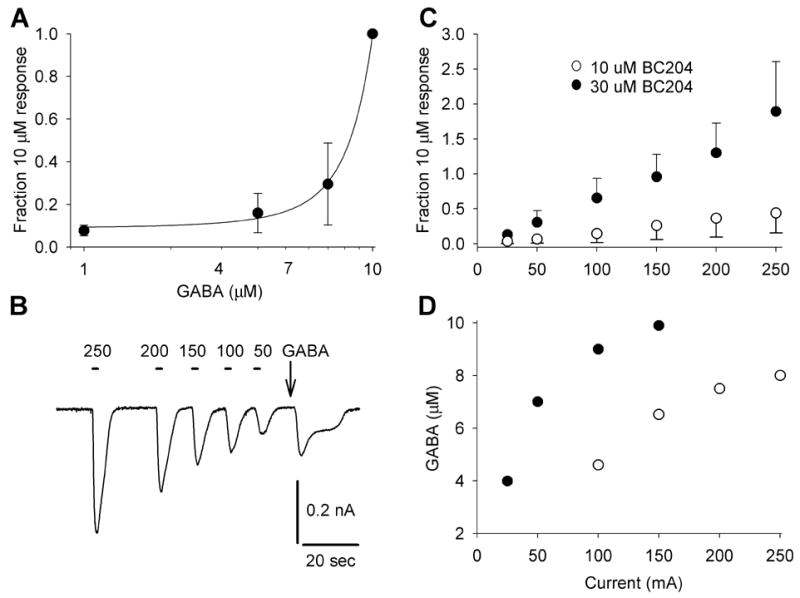
Concentration and current response relationships for GABA and BC204. A. Concentration response curve for GABA between 1 and 10 μM, normalized to the 10 μM response (n = 4–7 cells for each point). B. Increasing the current driving the UV LED (50 – 250 mA) causes an increase in inward current produced by BC204 (30 μM) uncaging. C. Inward, GABAergic current plotted as function of UV LED current and normalized to current produced by 10 μM GABA (n = 4 – 13 cells for each point). Data for both 10 and 30 μM BC204 are plotted. For 30 μM BC204, the current produced by LED current ≥ 150 mA exceeds the current produced by 10 μM GABA. D. Combining the data in A and B allow the GABA concentration produced by BC204 illumination to be estimated. LED currents below 100 mA produced GABA currents below the bottom of our dose response curve when we used 10 μM BC204. The abscissa is the same in C and D.
To verify that GABA was responsible for the neuronal currents induced by illumination of BC204 (30 μM), current responses in individual neurons in the presence and absence of the uncompetitive GABAA antagonist picrotoxin (100 μM) were compared. We found that picrotoxin reduced the inward currents by over 90% (p = 0.013 by paired t-test). There are reports that caged compounds can act as antagonists at the targeted receptor (Canepari et al., 2001;Maier et al., 2005). We, therefore, compared the responses of individual neurons (n = 13) to GABA (3 μM) in the presence and absence of BC204 (30 μM), to determine if BC204 influenced the magnitude of the GABA response in the absence of illumination. While there was some variability in the responses to bath perfused GABA, (possibly reflecting desensitization or run down), we did not observe any measurable antagonism of GABA by BC204, with the latter present in ten fold excess (p=0.126 by paired t-test).
3.2 Uncaging BC204 suppresses “seizure-like” activity
These preliminary experiments verified that our UV LED could induce robust GABAergic currents, even with only 10 μM BC204. There are a large number of reports of spontaneous paroxysmal, “seizure-like” activity in cultures of central neurons, and this activity can be amplified if cultures are exposed to an extracellular solution lacking magnesium (Gibbs et al., 1997;Wagenaar et al., 2005;Wagenaar et al., 2006). Subsequent experiments were focused on examining the effects of uncaging BC204 on “seizure-like” activity which appears when magnesium is removed from the perfusate.
When recording from cultured hippocampal neurons in the presence of extracellular calcium (2 mM) and magnesium (1 mM), there is often a low level of spontaneous activity (Figure 3A1 and 3B1). When the magnesium is removed, there is typically a dramatic increase in the rate of cell firing (Figure 3A2, 3A3, and 3B2). In some neurons, the firing is tonic and rapid enough to depolarize the neuron, while other cells develop phasic busting, with brief periods when the cell returns to resting potential. Occasionally, firing rates are unaffected, but large increases in synaptic activity that destabilize the resting potential are observed. This complex behavior has been recognized previously by others and has been the focus of recent quantitative analyses (Wagenaar et al., 2006). The spontaneous activity does not necessarily revert back to control levels when the magnesium concentration is normalized, because prolonged excessive firing can induce a permanent state of hyperexcitability in culture (Gibbs et al., 1997;Goodkin et al., 2005).
Figure 3.
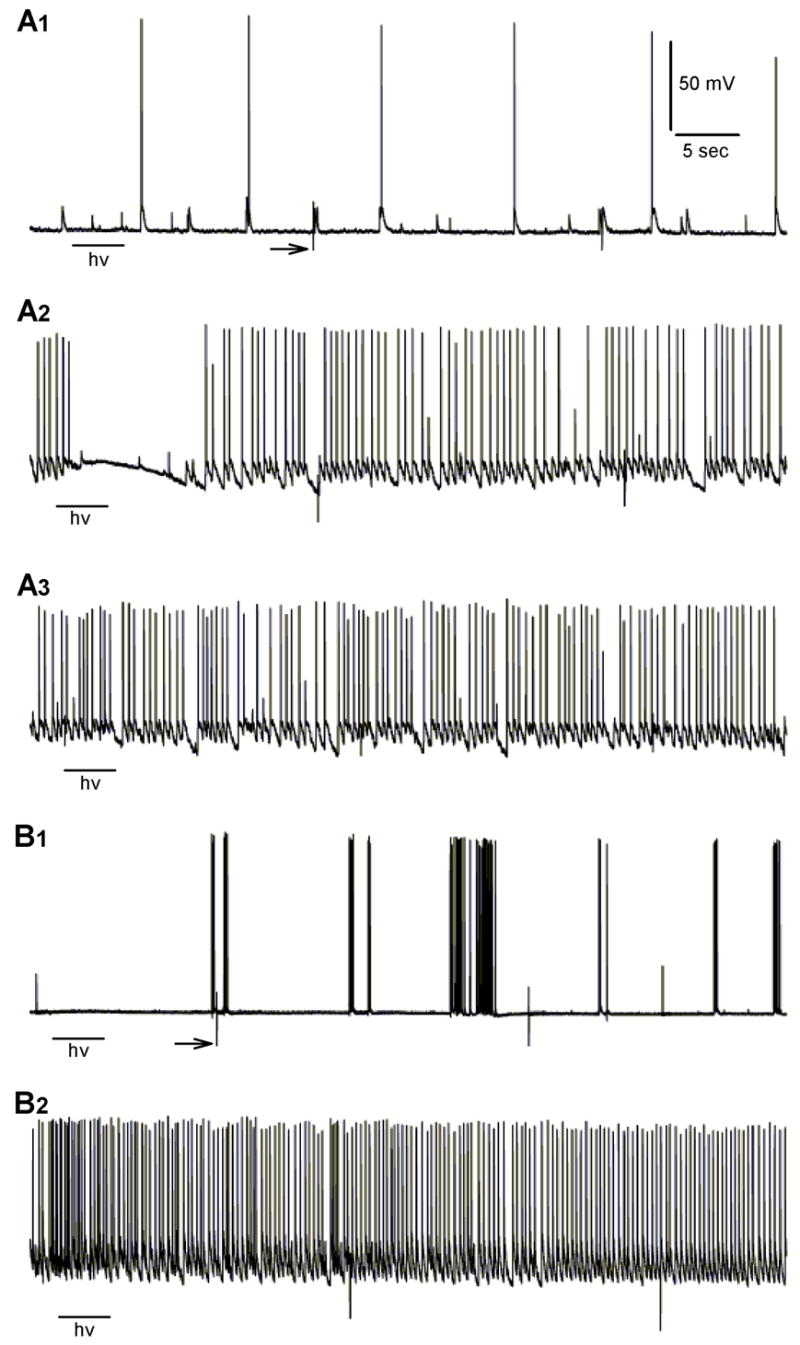
Examples of spontaneous activity and seizure-like events in two neurons under different experimental conditions. A1. Under control conditions (extracellular calcium and magnesium = 2 and 1 mM, respectively and no BC204), there were often some spontaneous postsynaptic potentials and action potentials. A 4 sec illumination with the UV LED (hv) had no effect on resting potential. A2. In the presence of BC204 (30 μM) and magnesium-free perfusate, UV illumination (200 mA for 4 sec) rapidly terminates the action potentials and quiets the voltage trace. A3. Lower power illumination (25 mA) has no effect on spiking or the baseline. B1. Under control conditions, another neuron shows some spontaneous bursting. B2. In the same cell, removing extracellular magnesium led to an abrupt, “seizure-like” increase in action potential firing that was unaffected by UV (200 mA) in absence of BC204. Scale in A1 applies to all traces. Arrows in A1 and B1 point to artifacts generated by the dish heater cycling.
When BC204 was added to the zero magnesium perfusate, there was no difference in the pattern of neuronal activity. However, when the UV LED was activated, there was a reduction or cessation of firing and cell membrane potential stabilized (Figure 3A2). The reduction in firing lasted for 10 – 20 sec, likely reflecting time required for the uncaged GABA to diffuse away from the recording site. Similarly, if exogenous GABA (3 – 10 μM) was added while the culture was bathed in zero magnesium, there was a marked reduction in firing and synaptic activity (not shown).
Although the effects of activated BC204 or GABA on seizure-like activity were qualitatively obvious, we developed a method to quantify our observations. We allowed 1 – 2 minutes for solution changes, and, in most experiments, illuminated for 4 sec, just at the onset of a 60 sec recording period. We examined the 10 sec epoch that included the last 2 sec of BC204 illumination plus the following 8 sec and measured the number of action potentials and the standard deviation of the baseline. We measured the same parameters in the final 10 sec of each epoch, which allowed any effect of light and uncaging to dissipate. When the same manipulation was repeated for a single cell, the measurements were averaged. When we examined 156 randomly selected epochs, we found that the two measures correlated (r2 = 0.66; Figure 4A). The regression line fails to intersect at the origin, because there are cells which have an elevated baseline standard deviation but no increase in action potentials.
Figure 4.
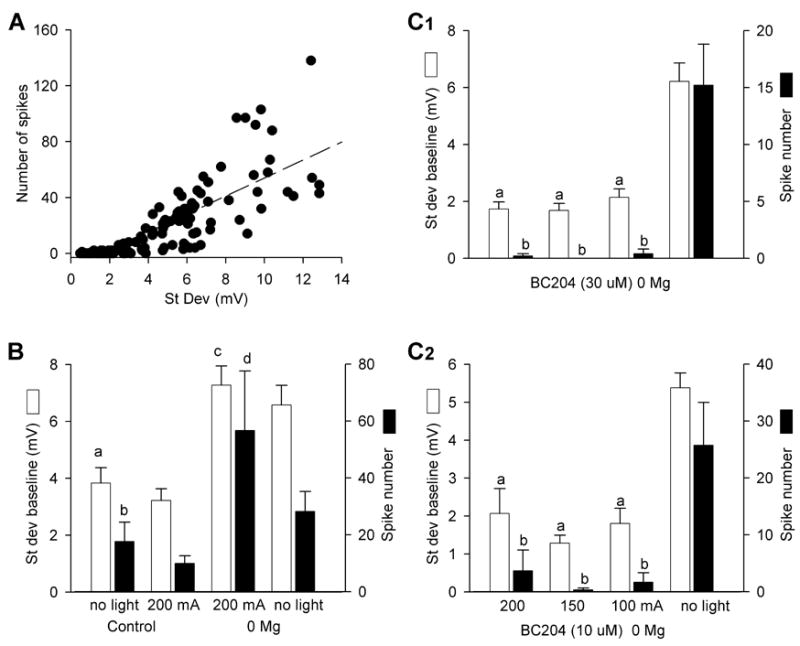
Quantification of seizure-like behavior in cultures. A. The number of spikes in a 10 sec interval was correlated with the standard deviation (mV) of the recording baseline (156 intervals; r2 = 0.66). B. In control and zero magnesium extracellular perfusate, UV LED illumination does not alter excitability in the absence of BC204 (a, b p = 0.25 and 0.46, respectively, compared to control plus light, n = 12–24 cells; c,d p = 0.39 and 0.10, respectively, compared to zero magnesium plus light, n = 6–17 cells, both by Student-Newman-Keuls test (SNK)). The larger baseline standard deviation and spike counts in the illuminated zero magnesium cultures reflects the variability in the seizure-like activity produced by magnesium withdrawal. Baseline standard deviations and spike numbers were significantly different between control and zero magnesium groups (p < 0.001 and p < 0.05, respectively by SNK). C1. In the presence of BC204 (30 μM) and zero magnesium, UV LED illumination using 200, 150, or 100 mA of current significantly reduced baseline standard deviation and spike number compared to 10 sec epochs in which there was no UV LED illumination (a p < 0.001 by SNK, compared to BC204 “no light”; b p < 0.05 by SNK, compared to BC204 “no light”, n = 9 – 11 cells). C2. In the presence of BC204 (10 μM) and zero magnesium, illumination with 200, 150, or 100 mA of UV LED current again significantly reduced both parameters compared epochs in which there was no UV LED illumination (a p < 0.001 by SNK, compared to “no light”; b p < 0.05 by SNK, compared to “no light”, n = 6 cells). C1 and C2 have the same abscissa. Bars in A –C are SEM.
When lower levels of current (25 – 100 mA) were used to activate the LED, illumination was prolonged from 4 to 8 sec. In those cases, we examined the last 4 sec of BC204 illumination plus the subsequent 6 sec, after the light was shut off. As above, baseline standard deviation and spike number obtained over this interval were compared to the same parameters measured over the final 10 sec of the 60 sec trace. The GABA effect was quantified by measuring spike number and baseline standard deviation over the entire 60 sec epoch during which the culture was perfused with low magnesium solution or an identical solution containing GABA at 1,3, or 10 μM.
When cultures were illuminated with the UV LED, in control or zero magnesium extracellular solution, there was no alteration in firing pattern or baseline standard deviation (Figures 3 and 4B). However, when we exposed the cultures to zero magnesium extracellular solution containing 30 μM BC204, there was a marked decrease in the standard deviation of the baseline and the number of action potentials (Figure 4C1). This effect was evident with LED currents from 100 – 200 mA. When we reduced the BC204 concentration to 10 μM, we found an almost identical result (Figure 4C2). However, when we used 3 μM BC204, there was no longer any effect on baseline standard deviation or spike number (n = 6 cells; data not shown).
In a subsequent set of experiments, we further reduced the current powering the UV LED to 25 – 100 mA and prolonged illumination to 8 sec. We found that 100 and 50, but not 25 mA, significantly reduced both spike number and baseline standard deviation (p< 0.05 for spike number and baseline standard deviation with 50 and 100 mA v non illuminated; n=13 cells; data not shown).
When cultures were exposed to picrotoxin in the presence of BC204 (30 μM) and absence of magnesium, 200 mA of UV LED current had no effect on spikes or baseline standard deviation (n= 5 cells, data not shown). Direct addition of GABA to zero magnesium perfusate suppressed paroxysmal activity. At 10 μM GABA, both baseline standard deviation and spike number were significantly reduced (p<0.02 v zero magnesium for both parameters; n=9 cells). At 3 μM, only spike number reduction was significant (p<0.01; n = 9 cells). There was no detectable effect of 1 μM GABA on either parameter (n=10 cells; data not shown).
3.3 Seizure termination identified by fluorescence imaging
While the intracellular recording provided solid evidence that BC204 uncaging could suppress in vitro seizure-like behavior in individual neurons, we wanted an independent method of assessing BC204 efficacy that allowed noninvasive and simultaneous assessment of seizure-like activity in multiple neurons. Neocortical cultures were loaded with the calcium-sensitive indicator Fluo-3 for fluorescence imaging. We expected that seizure-like activity, produced by exposing the cultures to zero magnesium would increase neuronal ionized intracellular calcium levels (Abele et al., 1990;Peterlin et al., 2000). While this type of imaging is too slow to temporally resolve single action potentials, it allows multiple cells in a microscopic field to be simultaneously analyzed for sustained increases in firing rates. In control extracellular solution (CaCl2 = 2; MgSO4 =1, both mM), neurons had stable baseline levels of ionized calcium, with intermittent transients likely corresponding to the spontaneous bursts observed in mature cultures (Figure 5A). When extracellular magnesium was removed, there was a sustained elevation of ionized calcium, which still showed some oscillations due to fluctuations in the neuronal firing rate (Figure 5A). Most neurons in a field fired synchronously and the calcium elevation was not influenced by illumination with the UV LED.
Figure 5.
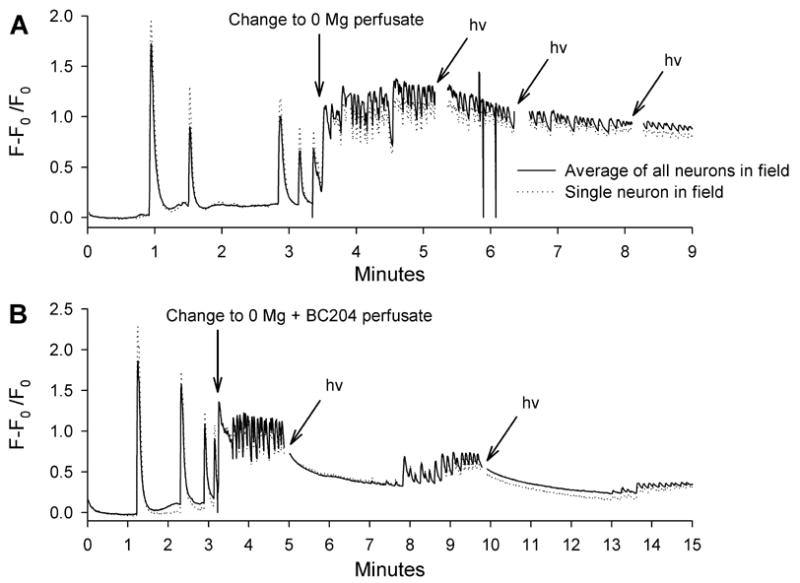
Effect of UV LED illumination on ionized intracellular calcium levels in neocortical cultures. Both traces show calcium peaks in normal extracellular magnesium, likely representing spontaneous bursting. Each figure shows averages of all neurons in a field (16 and 14, respectively for A and B) and then a single neuron, showing that the calcium elevations are synchronous A. Intracellular calcium levels rapidly rise and then oscillate when magnesium is removed from the extracellular solution. Three separate applications of UV LED illumination (hν = 200 mA for 5 sec) fail to alter the oscillation of the calcium levels. B. Intracellular calcium levels begin to rise and oscillate at the time of magnesium removal and BC204 addition. However, two light applications (hv) dramatically reduced ionized calcium. In both A and B, there are gaps in the trace coinciding with illumination, because the UV LED emission spectra overlaps the filters used for Fluo-3.
When BC204 was added to the extracellular solution at the same time that magnesium was removed, ionized calcium behaved differently. There was still a sustained rise in calcium levels, but immediately after UV LED illumination, ionized calcium steadily declined and the oscillations disappeared (Figure 5B). When the calcium levels again began to increase, a second light application immediately lowered them and eliminated the rapid fluctuations (Figure 5B).
4. Discussion
The results of the experiments described here suggest that it may be possible to use optical methods to influence neuronal activity and terminate paroxysmal activity. Caged compounds have been creatively employed in a variety of neurobiological experiments for a quarter of a century and have provided kinetic information that would have been unattainable using only electrophysiological techniques (Lester and Nerbonne, 1982). However, in almost all of the previously reported work on caged compounds, either lasers or arc lamps were used to release the physiological ligand. Furthermore, almost all of the earlier studies were focused upon achieving very rapid “step” elevations in the local concentration of ligand, such that high concentrations of caged compounds and brief (msec), high intensity illumination were employed. Recent advances in solid state devices suggested the possibility of producing efficient uncaging with smaller, energy efficient UV LEDs (Schubert and Kim, 2005). Indeed, a recent paper describes the use of an LED identical to the one used here to release caged glutamate, although a 100 fold higher concentration of caged compound and much higher (2.5 fold) LED power were employed (Bernardinelli et al., 2005).
The contribution of extrasynaptic GABAA receptors to circuit behavior is probably critical in enabling caged GABA to exert an antiepileptic effect. Several recent reports have heightened awareness of relatively nondesensitizing, high affinity GABAA receptors that are present on dendrites at some distance from the sites of transmitter release (Farrant and Nusser, 2005;Kullmann and Asztely, 1998;Nusser et al., 1998;Nusser and Mody, 2002;Scimemi et al., 2005;Semyanov et al., 2004). These receptors are gated by the low concentrations of GABA spilling out of the synaptic cleft and by the GABA normally present in the extracellular space. While the absolute level of charge transfer gated by these receptors is small at any instant in time, their total contribution to chloride flux is higher than the contribution from phasically activated synaptic receptors. Even though it might be possible to activate these receptors directly by local perfusion of GABA or a GABA analog, avid GABA uptake and poor temporal control over onset of action will make this method of application very unreliable. Production of GABA from a caged compound under optical control will generate a more dependable elevation in local extracellular GABA levels. There is still no absolute assurance that activation of tonic GABAA receptors will invariably be antiepileptic, because their activation facilitates bursting in some parts of the brain (Cope et al., 2005).
It is, of course, possible that some of the antiepileptic effect of uncaging BC204 is due to activation of metabotropic or GABAB receptors, which also have high affinity for GABA (Yoon and Rothman, 1991). The complete block of the antiepileptic effect of BC204 by picrotoxin makes this unlikely, but it remains possible that uncaged GABA acts at both post- and presynaptic sites.
At this time, we have not demonstrated the feasibility of our ideas in a truly realistic model of epilepsy. However, it seemed important to us to obtain proof of principle in a more tractable in vitro system. Other investigators have utilized neuronal cultures as reasonable models for seizures, and the spontaneous or provoked bursting that we all observe resembles the pathological activity seen in intracellular recordings from epileptic foci (Goodkin et al., 2005;Prince and Connors, 1984;Wagenaar et al., 2005). The complete withdrawal of extracellular magnesium is an especially severe provocation. It facilitates neurotransmitter release, removes the voltage-dependent blockade of NMDA receptors, and directly destabilizes excitable membranes by eliminating the screening effects of divalent cations (Frankenhaeuser et al., 1957;Katz and Miledi, 1967;Mayer et al., 1984;Nowak et al., 1984). It, therefore, creates a more robust form of pathological activity than human metabolic seizures, which are precipitated by less drastic alterations in electrolyte concentration. The ability of uncaged BC204 to quickly quiet bursts makes us optimistic that this technique may translate to the treatment of real epilepsy. BC204’s brief duration of efficacy in our culture system is not much of a surprise, because the neurons remain in zero magnesium after the uncaged GABA is taken up or diffuses away.
It would be misleading to suggest that the techniques that we have employed in these tissue culture experiments are ready for in vivo application today. However, it is possible to identify many of the practical issues that need to be addressed to allow development of a device that would help control epilepsy in humans. The first critical component of any system is the actual caged compound. BC204 worked extremely well in our cultures. When it was initially characterized in brain slice experiments, it was stable in tissue for at least two hours (duration of experiment) (Curten et al., 2005). Furthermore, it remained stable in solution for at least 11 days. Neither the initial slice experiments, which used BC204 at 200 μM, nor ours detected any physiological activity of the uncaged compound (Curten et al., 2005). There is still some concern that BC204 could be a very weak GABA antagonist, but we have not detected this effect so far. We have not exhaustively searched for other possible interactions between BC204 and different receptors or channels. Similarly, we have no notion about the possible toxicity of this compound. It is not unreasonable at this point to believe that BC204, or a related compound, could be safely administered to humans.
The route of administration of a drug that would eventually require access to the brain extracellular space needs to be considered. Under the best of circumstances, it might be absorbed after oral administration and cross the blood-brain barrier. However, even if promising caged compounds were unable to cross into brain, they could be administered directly into the subarachnoid space by pump and catheter. This agonist baclofen, in order to achieve high concentrations is already done for the GABAB in the spinal subarachnoid space (Albright et al., 2006). The pump technology could be modified to allow infusions into the cranial subarachnoid space overlying epileptogenic neocortex.
There may already be an adequate UV LED light source for in vivo uncaging. The actual diode element of the LED used in our experiments is only about 1 mm2. It will be possible to configure a single diode or an array on a silastic grid, similar to the grids now used to mount the recording electrodes used for cortical mapping during epilepsy surgery. The grid would be positioned so that the LEDs were located over the epileptic focus. There is already a report of a similar application of a miniature LED for in vitro uncaging, so this is a realistic technical expectation (Venkataramani et al., 2006). Moreover, newer UV LEDs, with identical spectral characteristics and twice the optical power of the device used in these experiments, are now commercially available.
We anticipate powering the LEDs for only a few seconds, so heat dissipation may not be a problem. If this assumption is incorrect, we have independently worked on developing thin, flexible heat pipes for medical applications, so we may be able to channel heat to the vascular dura or skull (S. Rothman, manuscript in press).
A bigger problem is the depth of penetration of UV light through the arachnoid and underlying cortex. We have preliminary results suggesting that about 50% of 365 nm light is absorbed over each 200 μm, so that there will be substantial light attenuation within the first mm of cortex. However, we detected an effect of BC204 at current levels generating only 10% of the maximum UV LED power, so positioning an LED at the cortical surface could still transmit adequate light to a depth of a few hundred μm. Furthermore, a fiber optic guide could move light even closer to the epileptogenic zone. While inserting a thin fiber into cortex makes our technique more invasive, it would still be preferable to irreversible resection.
A related concern is the possibility of toxicity of UV light on brain or direct interactions of UV light with other receptors or channels. There should not be too much concern about direct toxic effects of 365 nm light, because the UV LED will not be activated for long durations. There are some provocative reports suggesting direct effects of UV on GABA and NMDA receptors. However, in both cases the offending light had to be < 340 nm, well below the spectral peak of our UV LED (Chang et al., 2001;Leszkiewicz et al., 2000).
There are two ways we envision the control of uncaging. The LED could be activated tonically to suppress activity in an epileptogenic region of cortex. Alternately, it might be possible to use a seizure detection program to put the UV LED under closed loop control. Such a program, combined with rapid electrical stimulation, has been developed for a totally implantable device and is currently in clinical trials (Fountas et al., 2005;Morrell, 2006). Given the proliferation of successful implantable medical devices over the last decade and recent interest in the optical control of neurons by other investigators, LED uncaging of GABA does not represent a radical deviation from the current trajectory of medical care (Banghart et al., 2004;Kramer et al., 2005;Miller, 2006). The neurobiology underlying our ideas is grounded by a substantial experimental literature, and there are few good therapeutic options available for many patients with intractable focal epilepsy. This one merits exploration in epilepsy models that more closely mimic the human disease.
Acknowledgments
We would like to thank Nicholas Rensing for preparing the hippocampal cultures and Joy Snider, MD, PhD for allowing us to use several neocortical cultures for the calcium imaging. Dr Jeanne Nerbonne offered critical experimental advice and made many suggestions to improve the final manuscript. This work was supported by the Alafi Family Foundation and the NIH (R01 NS42936 to SMR and P01 NS NS32636 to Mark Goldberg, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abele AE, Scholz KP, Scholz WK, Miller RJ. Excitotoxicity induced by enhanced excitatory neurotransmission in cultured hippocampal pyramidal neurons. Neuron. 1990;4:413–419. doi: 10.1016/0896-6273(90)90053-i. [DOI] [PubMed] [Google Scholar]
- Albright AL, Turner M, Pattisapu JV. Best-practice surgical techniques for intrathecal baclofen therapy. J Neurosurg. 2006;104:233–239. doi: 10.3171/ped.2006.104.4.233. [DOI] [PubMed] [Google Scholar]
- Alpherts WC, Vermeulen J, van Rijen PC, da Silva FH, van Veelen CW. Verbal memory decline after temporal epilepsy surgery?: A 6-year multiple assessments follow-up study. Neurology. 2006;67:626–631. doi: 10.1212/01.wnl.0000230139.45304.eb. [DOI] [PubMed] [Google Scholar]
- Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardinelli Y, Haeberli C, Chatton JY. Flash photolysis using a light emitting diode: an efficient, compact, and affordable solution. Cell Calcium. 2005;37:565–572. doi: 10.1016/j.ceca.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Canepari M, Nelson L, Papageorgiou G, Corrie JE, Ogden D. Photochemical and pharmacological evaluation of 7-nitroindolinyl-and 4-methoxy-7-nitroindolinyl-amino acids as novel, fast caged neurotransmitters. J Neurosci Methods. 2001;112:29–42. doi: 10.1016/s0165-0270(01)00451-4. [DOI] [PubMed] [Google Scholar]
- Chang Y, Xie Y, Weiss DS. Positive allosteric modulation by ultraviolet irradiation on GABA(A), but not GABA(C), receptors expressed in Xenopus oocytes. J Physiol. 2001;536:471–478. doi: 10.1111/j.1469-7793.2001.0471c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curten B, Kullmann PH, Bier ME, Kandler K, Schmidt BF. Synthesis, photophysical, photochemical and biological properties of caged GABA, 4-[[(2H-1-benzopyran-2-one-7-amino-4-methoxy) carbonyl] amino] butanoic acid. Photochem Photobiol. 2005;81:641–648. doi: 10.1562/2004-07-08-RA-226. [DOI] [PubMed] [Google Scholar]
- Eder HG, Jones DB, Fisher RS. Local perfusion of diazepam attenuates interictal and ictal events in the bicuculline model of epilepsy in rats. Epilepsia. 1997;38:516–521. doi: 10.1111/j.1528-1157.1997.tb01134.x. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fountas KN, Smith JR, Murro AM, Politsky J, Park YD, Jenkins PD. Implantation of a closed-loop stimulation in the management of medically refractory focal epilepsy: a technical note. Stereotact Funct Neurosurg. 2005;83:153–158. doi: 10.1159/000088656. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser B, Hodgkin A. The action of calcium on the electrical properties of squid axon. J Physiol. 1957;137:218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JW, Sombati S, Delorenzo RJ, Coulter DA. Physiological and pharmacological alterations in postsynaptic GABA(A) receptor function in a hippocampal culture model of chronic spontaneous seizures. Journal of Neurophysiology. 1997;77:2139–2152. doi: 10.1152/jn.1997.77.4.2139. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25:5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The timing of calcium action during neuromuscular transmission. J Physiol. 1967;189:535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer RH, Chambers JJ, Trauner D. Photochemical tools for remote control of ion channels in excitable cells. Nat Chem Biol. 2005;1:360–365. doi: 10.1038/nchembio750. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F. Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. Trends Neurosci. 1998;21:8–14. doi: 10.1016/s0166-2236(97)01150-8. [DOI] [PubMed] [Google Scholar]
- Lester HA, Nerbonne JM. Physiological and pharmacological manipulations with light flashes. Annu Rev Biophys Bioeng. 1982;11:151–175. doi: 10.1146/annurev.bb.11.060182.001055. [DOI] [PubMed] [Google Scholar]
- Leszkiewicz DN, Kandler K, Aizenman E. Enhancement of NMDA receptor- mediated currents by light in rat neurones in vitro. J Physiol. 2000;524(Pt 2):365–374. doi: 10.1111/j.1469-7793.2000.t01-1-00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Corrie JE, Papageorgiou G, Laube B, Grewer C. Comparative analysis of inhibitory effects of caged ligands for the NMDA receptor. J Neurosci Methods. 2005;142:1–9. doi: 10.1016/j.jneumeth.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Miller G. Optogenetics. Shining new light on neural circuits. Science. 2006;314:1674–1676. doi: 10.1126/science.314.5806.1674. [DOI] [PubMed] [Google Scholar]
- Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol. 2006;19:164–168. doi: 10.1097/01.wco.0000218233.60217.84. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABA(A) receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. Journal of Neuroscience. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin ZA, Kozloski J, Mao BQ, Tsiola A, Yuste R. Optical probing of neuronal circuits with calcium indicators. Proc Natl Acad Sci U S A. 2000;97:3619–3624. doi: 10.1073/pnas.97.7.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DA, Connors BW. Mechanisms of epileptogenesis in cortical structures. Ann Neurol. 1984;16(Suppl):S59–S64. doi: 10.1002/ana.410160710. [DOI] [PubMed] [Google Scholar]
- Rausch R, Kraemer S, Pietras CJ, Le M, Vickrey BG, Passaro EA. Early and late cognitive changes following temporal lobe surgery for epilepsy. Neurology. 2003;60:951–959. doi: 10.1212/01.wnl.0000048203.23766.a1. [DOI] [PubMed] [Google Scholar]
- Schubert EF, Kim JK. Solid-state light sources getting smart. Science. 2005;308:1274–1278. doi: 10.1126/science.1108712. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol. 2004;3:111–118. doi: 10.1016/s1474-4422(03)00664-1. [DOI] [PubMed] [Google Scholar]
- Thio LL, Zhang HX. Modulation of inhibitory glycine receptors in cultured embryonic mouse hippocampal neurons by zinc, thiol containing redox agents and carnosine. Neuroscience. 2006;139:1315–1327. doi: 10.1016/j.neuroscience.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Venkataramani S, Davitt KM, Xu H, Zhang J, Song YK, Connors BW, Nurmikko AV. Semiconductor ultra-violet light-emitting diodes for flash photolysis. J Neurosci Methods. 2006 doi: 10.1016/j.jneumeth.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Wagenaar DA, Madhavan R, Pine J, Potter SM. Controlling bursting in cortical cultures with closed-loop multi-electrode stimulation. J Neurosci. 2005;25:680–688. doi: 10.1523/JNEUROSCI.4209-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar DA, Pine J, Potter SM. An extremely rich repertoire of bursting patterns during the development of cortical cultures. BMC Neurosci. 2006;7:11. doi: 10.1186/1471-2202-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Rothman SM. Focal cooling rapidly terminates experimental neocortical seizures. Annals of Neurology. 2001;49:721–726. doi: 10.1002/ana.1021. [DOI] [PubMed] [Google Scholar]
- Yoon KW, Rothman SM. The modulation of rat hippocampal synaptic conductances by baclofen and gamma-aminobutyric acid. Journal of Physiology (London) 1991;442:377–390. doi: 10.1113/jphysiol.1991.sp018798. [DOI] [PMC free article] [PubMed] [Google Scholar]


