Abstract
Pyrrolysine (Pyl) is co-translationally inserted into a subset of proteins in the Methanosarcinaceae and in Desulfitobacterium hafniense programmed by an in-frame UAG stop codon. Suppression of this UAG codon is mediated by the Pyl amber suppressor tRNA, tRNAPyl, which is aminoacylated with Pyl by pyrrolysyl-tRNA synthetase (PylRS). We compared the behavior of several archaeal and bacterial PylRS enzymes towards tRNAPyl. Equilibrium binding analysis revealed that archaeal PylRS proteins bind tRNAPyl with higher affinity (Kd=0.1–1.0 µM) than D. hafniense PylRS (Kd=5.3–6.9 µM). In aminoacylation the archaeal PylRS enzymes did not distinguish between archaeal and bacterial tRNAPyl species, while the bacterial PylRS displays a clear preference for the homologous cognate tRNA. We also show that the amino-terminal extension present in archaeal PylRSs is dispensable for in vitro activity, but required for PylRS function in vivo.
Keywords: Pyrrolysine, pyrrolysyl-tRNA synthetase, tRNA, aminoacyl-tRNA synthetase
1. Introduction
Pyrrolysyl-tRNA synthetase is unique among the aminoacyl-tRNA synthetases (aaRSs), as it mediates the co-translational insertion of the unusual amino acid pyrrolysine into proteins [1, 2]. While the 20 canonical amino acids used in protein synthesis are genetically encoded by 61 sense codons, Pyl has been shown to be inserted into the methylamine methyltransferases of Methanosarcina barkeri and Methanosarcina acetivorans in response to an in-frame UAG stop codon embedded in the corresponding mRNAs [3, 4]. The decoding of these particular UAG codons as Pyl is achieved by the presence of pylT that encodes the pyrrolysine-specific amber suppressor tRNAPyl [5]. Comparative genomic analyses have shown that closely related orthologs of pylT and pylS, the gene encoding PylRS, are present in the genomes of the other members of the Methanosarcinaceae family (Methanosarcina mazei and Methanococcoides burtonii). While sequence similarity implies these orthologs have similar function, no biochemical data exist establishing their enzymatic activity. In addition to the Methanosarcinaceae, pylT and pylS are present in the strictly anaerobic bacterium D. hafniense [5]. PylRS from this organism has been shown to aminoacylate tRNAPyl in vitro [6].
Here we report the amino acid sequence and enzymatic activity of PylRS from an additional member of the Methanosarcinaceae, Methanosarcina thermophila, and compare the affinities and aminoacylation preference of three of the Methanosarcinaceae PylRSs and of the D. hafniense PylRS toward the known tRNAPyl species. Finally, we investigate the potential role of the archaeal PylRS amino-terminal domain..
2. Materials and methods
2.1 General
Oligonucleotide synthesis and DNA sequencing were performed by the Keck Foundation Biotechnology Resource Laboratory at Yale University. Uniformly labeled sodium [32P]pyrophosphate [1–60 Ci/mmol (1 Ci = 37 GBq)], and [α-32P]ATP (10 mCi/ml) was from Amersham Biosciences. N-ε-cyclopentyloxycarbonyl-L-lysine (Cyc) was from Sigma.
2.2 Cloning and purification of recombinant PylRS enzymes
As the M. thermophila genome sequence is not available, pylS and pylT genes were amplified from genomic DNA by PCR assuming reasonable levels of sequence identity of upstream and downstream regions with other sequenced Methanosarcinaeae. The amplified DNA was cloned into the pCR2.1–TOPO vector (Invitrogen) and sequenced multiple times. M. thermophila pylS nucleotide sequence was deposited in GenBank (accession number DQ017250). M. thermophila pylS was subcloned into pET15b vector (Novagen) using NdeI and BamHI as restriction sites. The M. acetivorans pylS gene was amplified by PCR based on the available genomic sequence, cloned into the pCR2.1–TOPO vector (Invitrogen), sequenced, and subcloned into pET15b vector (Novagen) using NdeI and BamHI as restriction sites. The M. barkeri pylS (Fusaro and MS strains) and D. hafniense pylS expression constructs were as described [2,6]. All clones were transformed into Escherichia coli BL21-(DE3)-RIL (Stratagene). The overexpression and purification of the recombinant His6-PylRS enzymes was performed as described [2,6].
2.3 Cloning of M. barkeri Fusaro and D. hafniense pylS and pylT genes for in vivo activity assay
The D. hafniense pylS gene was amplified by PCR, cloned into the pCR2.1–TOPO vector (Invitrogen), sequenced, and subcloned into pCBS (Ampr), using NdeI and KpnI as restriction sites. Full length and amino-terminal truncations of M. barkeri Fusaro pylS were generated by PCR with primers corresponding to the desired length. The PCR products were cloned into the pCR2.1–TOPO vector (Invitrogen), sequenced, and subcloned into pCBS vector using NdeI and XhoI as restriction sites. The truncation sites are: Δ1–16 (nucleotide 49, protein starts at M17), Δ1–30 (nucleotide 91, protein starts at M31), Δ1–73 (nucleotide 220, protein starts at M74), Δ1–106 (nucleotide 319, protein starts at M107). Point mutations in the pylS gene were inserted by PCR with the Quick Change Mutagenesis kit (Qiagen) using the wild type M. barkeri Fusaro pylS, cloned into pCBS as a template. The constructs were entirely sequenced in order to verify that the desired mutation had been obtained and that no other mutations had been inserted during PCR. The D. hafniense and M. barkeri Fusaro pylT genes were cloned into the pTECH vector as described [12].
2.4 Cloning and in vitro transcription of the tRNAPyl substrates
Transcripts of M. barkeri (strains Fusaro and MS), M. burtonii and D. hafniense tRNAPyl were prepared and 3′ end-labeled as reported [6]. In vitro T7 RNA polymerase run off transcription was conducted according to standard procedures [7].
2.5 Filter binding assay
The ability of the PylRSs to bind [α-32P]ATP 3′-labeled tRNAPyl transcript across species was determined using a standard filter binding assay [8]. 3′-labeled tRNA bound to PylRS (tRNAbound) was captured on a nitrocellulose membrane (Millipore); unbound 3′-labeled tRNA (tRNAfree) was captured on a nylon Hybond N+ membrane (Amersham). The binding of tRNAbound and tRNAfree were quantified and used to calculate the binding curve for Kd determination. The Kd of the PylRSs for the various tRNAPyl were determined by fitting the data to a simple binding isotherm: θ=[enzyme]/([enzyme]+Kd) [9].
2.6 ATP-[32P]PPi exchange
The assay was used to measure PylRS activation of amino acid substrates, and performed as described [10]. Reactions (200 µl final volume) were carried out at 37 °C (50 °C for M. thermophila PylRS) in 100 mM Na-Hepes, pH 7.2, 10 mM MgCl2, 50 mM KCl, 5 mM DTT, 2 mM KF, 2 mM ATP, 2 mM [32P]PPi (1.6 cpm/pmol), 0.5 µM PylRS enzyme and either, 10 mM Cyc, 1 mM lysine or no amino acid. Adsorption of formed [32P]ATP on acid-washed Norite, filtration, wash and quantification were performed as described [10].
2.7 PylRS aminoacylation of tRNAPyl with Cyc
Aminoacylation assays were adapted from a recently described procedure [11]. Aminoacylation reactions (10 µl) were carried out at 37 °C (50 °C for M. thermophila PylRS) in 100 mM Na-Hepes, pH 7.2, 25 mM MgCl2, 60 mM NaCl, 5 mM ATP, 1 mM DTT, 10 mM Cyc, 5 nM PylRS and 1 µM 3′-[α-32P]ATP labeled tRNAPyl. Nuclease P1 digest, thin layer chromatography, and quantification were performed as described [6].
2.8 Suppression of E. coli XAC/A24 lacI-lacZ nonsense mutation
In vivo suppression experiments were carried out using E. coli strain XAC/A24 as described [12]. E. coli strain XAC/A24 cells were co-transformed with plasmids carrying the M. barkeri Fusaro pylT (pTECH) and M. barkeri Fusaro wild type pylS or any of its variants (pCBS). E. coli strain XAC/A24 cells were also co-transformed with D. hafniense pylT (pTECH) and D. hafniense pylS (pCBS). The UAG suppression level was determined by quantitative analysis of the β-galactosidase activity, which was performed according to the standard procedure [13]. Values are the average of triplicate measurements and are reported as the percentage of mutant enzyme activity relative to that of the wild type enzyme produced by the E. coli I-Z40 strain, which carries the lacI-lacZ fusion with a wild type tryptophan codon in place of the UAG triplet.
2.9 In vivo binding affinity of tRNAPyl for PylRS using the yeast three hybrid system
The yeast three hybrid experiments were performed as described [21]. Effect of mutations in PylRS on tRNAPyl binding was measured using the in vivo yeast three hybrid method as reported [14].
2.10 Acid urea gel electrophoresis of aminoacyl-tRNA and Northern hybridization
Acid urea gel electrophoresis and Northern blot were performed as described [2]. The aminoacylation levels of tRNAPyl isolated from the various E. coli XAC/A24 strains transformed with D. hafniense pylS, M. barkeri Fusaro pylS wild type or its variants, grown in the presence of 10 mM Cyc was determined.
3. Results
3.1 Sequence variations in PylRS orthologs
The pylS and pylT sequences from the methanogenic archaea M. barkeri, M. acetivorans, M. mazei and M. burtonii as well as from the bacterium D. hafniense are available in the public sequence databases. Recently, a metagenomic analysis revealed an additional bacterial pylS homologue that is present in a δ-proteobacterial member of the marine worm Olavius algarvensis endosymbiotic community [15]. M. thermophila, another organism from the Methanosarcinaceae group, has been the object of many biochemical studies [16]; its genome sequence, however, has not yet been reported. In order to gain more insight on PylRS sequence variations, we cloned and determined the nucleotide sequence of the M. thermophila pylS gene (GenBank accession number Q1L6A3) (Fig. S1).
PylRS sequences can be subdivided into three regions: the highly conserved class II aaRS signature core domain at the carboxy-terminal, the unique amino-terminal domain, and a highly variable region linking these two domains. The linking region in archaeal PylRS differs substantially in sequence and in length. This region is relatively short in M. barkeri (16 aa) and M. burtonii (14 aa) PylRSs compared to the much longer linkers present in M. thermophila (74 aa), M. mazei (50 aa), and M. acetivorans (42 aa) PylRS proteins. In M. thermophila, the region is composed of five degenerate repeats of the PAPASTTA sequence (Fig. S1). The PylRSs of the Methanosarcinaceae range from 52%–75% sequence identity, and from 56%–82% when the linking region is excluded. Taken together, the other aaRSs from this taxonomic group show a slightly wider range of variability (57%–94% sequence identity).
The bacterial PylRSs from D. hafniense and the O. algarvensis endosymbiotic δ-proteobacteria differs markedly from the archaeal PylRSs, most notably through the absence of approximately 100 amino acids from their amino-terminal ends. Overall, the bacterial PylRSs share 23%–30% sequence identity with their archaeal counterparts. BLAST searches in the two bacterial genomes identified a distinct short open reading frame (pylSn) homologous to the archaeal amino-terminal domain downstream of pylS [5]. The bacterial PylSn sequences share 59% identity to each other, and ~33–39% identity to the amino-terminus of the archaeal PylRS consensus sequence (Fig. S1).
The five Methanosarcinaceae and D. hafniense have the same genomic organization of their pyl genes: pylT is directly upstream of pylS which is followed by three genes called pylB, pylC and pylD, whose products are involved in Pyl biosynthesis [17]. In D. hafniense, pylSn is found downstream of pylD [5]. In the worm endosymbiotic δ-proteobacterium, pylSn is found immediately downstream of pylS while pylB, pylC and pylD are located in a separate cluster elsewhere in the genome.
The archaeal tRNAPyl species are more conserved than their corresponding PylRSs. All Methanosarcinaceae tRNAPyl have identical or nearly identical nucleotide sequences (Fig. 1 and Fig. S1). In contrast, the D. hafniense tRNAPyl contains 23 nucleotide changes when compared to the archaeal consensus sequence (Fig. S1,[6]). Searches in genomes of the worm endosymbiotic bacterial community either by BLAST or using tRNA Scan-SE with various settings revealed the presence of a possible amber tRNA suppressor. However, this putative tRNA lacks the distinctive secondary structure of pylT and includes a number of mismatches in the acceptor stem (Fig. S1).
Fig. 1.
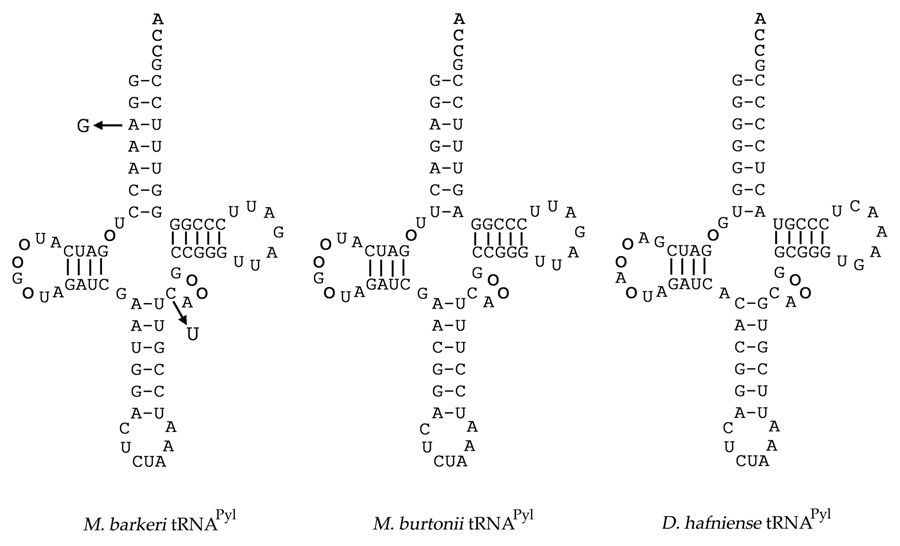
Clover leaf structures of tRNAPyl from M. barkeri Fusaro, M. burtonii, and D. hafniense. Arrows indicate nucleotide variations in M. barkeri MS tRNAPyl. Circles represent absent nucleotides according to standard tRNA numbering [23].
3.2 Archaeal PylRS has a higher affinity for tRNAPyl than D. hafniense PylRS
An equilibrium binding analysis was carried out to determine the relative PylRS affinities for the various tRNAPyl species. All archaeal PylRS enzymes bound both the archaeal and D.hafniense tRNAPyl transcripts with a Kd ranging from 0.15–1 µM (Table 1). While M. acetivorans PylRS did not show any preference towards either the D. hafniense or archaeal tRNAPyl, M. thermophila and M. barkeri Fusaro PylRS bound the archeal tRNAPyl slightly better than the D. hafniense tRNAPyl (Table 1). Overall, the nucleotide differences between the archaeal and D. hafniense tRNAPyl did not have a major effect on binding by the archaeal synthetases. The D. hafniense PylRS bound tRNAPyl with a lower affinity relative to the archaeal enzymes with Kd ranging from 5.3–6.9 µM (Table 1). The overall lower affinity of the D. hafniense PylRS toward tRNAPyl could be due to the absence of the amino-terminal domain. All tested PylRSs were able to bind tRNAPyl specifically irrespective of the presence or absence of total yeast RNA (0.5 µg/µl) used as competitor in the reaction mixture.
Table 1.
Dissociation constants (Kd, µM) of archaeal and bacterial PylRSs for all known tRNAPyl species as determined by nitrocellulose filter binding assay.
| Enzymes | D. hafniense tRNAPyl | M. acetivorans tRNAPyl* | M. barkeri MS tRNAPyl | M. burtonii tRNAPyl |
|---|---|---|---|---|
| M. barkeri Fusaro PylRS | 0.98 ± 0.07 | 0.32 ± 0.02 | 0.15 ± 0.02 | 0.43 ± 0.03 |
| M. thermophila PylRS | 1.00 ± 0.05 | 0.71 ± 0.06 | 0.16 ± 0.01 | 0.19 ± 0.02 |
| M. acetivorans PylRS | 0.50 ± 0.08 | 0.62 ± 0.02 | 0.25 ± 0.01 | 0.44 ± 0.03 |
| D. hafniense PylRS | 6.90 ± 0.41 | 6.50 ± 0.26 | 5.40 ± 0.18 | 5.30 ± 0.37 |
The sequences of tRNAPyl from M. barkeri Fusaro, M. thermophila, and M. acetivorans are identical.
3.3 All PylRSs activate the Pyl analogue Cyc
Pyrrolysine is not a commercially available substrate, and its synthesis has proven both difficult and expensive [2, 12, 18]. The pyrrolysine analog, N-ε-cyclopentyloxycarbonyl-L-lysine (Cyc), is a reasonable substrate for M. barkeri Fusaro PylRS in vitro and in vivo, and for D. hafniense PylRS in vitro [6,12]. Here, the ability of M. barkeri (MS and Fusaro strains), M. acetivorans and M. thermophila PylRS enzymes to activate Cyc and promote the ATP-[32P]PPi exchange reaction was assayed. All tested PylRSs were able to activate Cyc while rejecting lysine (Figure 2A).
Fig. 2.
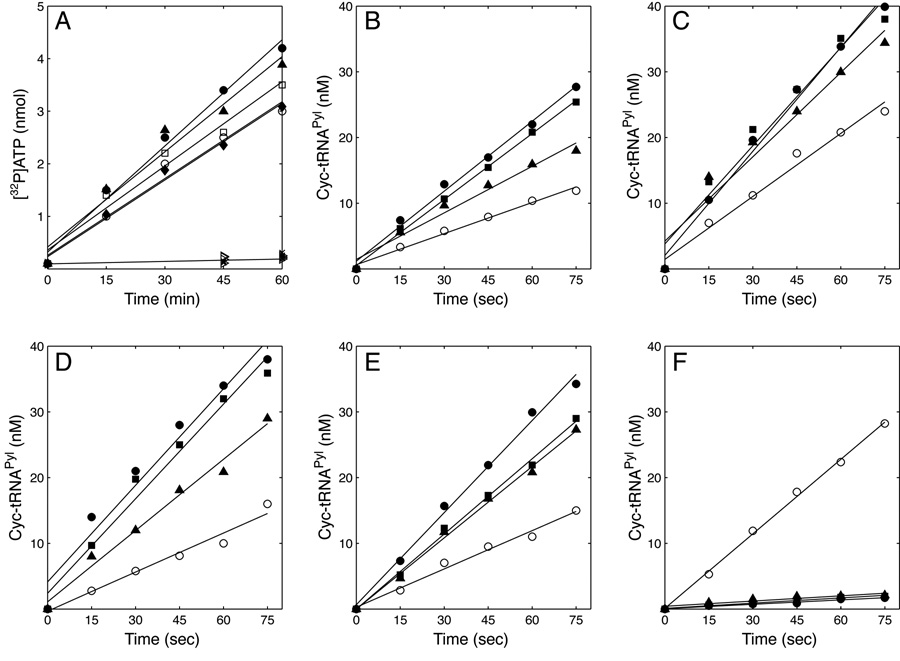
Activation and charging properties of various PylRSs. (A) Time course for the activation of Cyc by PylRS from M. barkeri Fusaro (♦), M. barkeri MS (▲), M. thermophila (●), M. acetivorans (□), and D. hafniense (○). All PylRS proteins were assayed in reactions with lysine (×) and no amino acid (▹), and no activation was observed. In vitro aminoacylation of tRNAPyl transcript with Cyc by PylRS from: (B) M. barkeri Fusaro, (C) M. barkeri MS, (D) M. acetivorans, (E) M. thermophila, (F) D. hafniense. tRNAPyl was from M. barkeri MS (■), M. barkeri Fusaro (●), M. burtonii (▲), and D. hafniense (○). Reaction conditions are described in Materials and Methods.
3.4 All PylRSs aminoacylate tRNAPyl with Cyc
Cyc was used to assay the ability of PylRS to aminoacylate tRNAPyl transcripts in vitro (Figure 2B-F) using the established Wolfson/Uhlenbeck assay [6,11]. Each of the different PylRS enzymes was shown to aminoacylate the entire set of tRNAPyl species in vitro. However, there were variations in aminoacylation rates depending on the enzyme:tRNA pair (Figure 2B-F). The archaeal enzymes showed no preference toward any of the various archaeal tRNAPyl species. Despite the sequence differences, the archaeal enzymes charged D. hafniense tRNAPyl with only a small decrease in the aminoacylation rate relative to the archaeal tRNAPyl species.
In contrast, the D. hafniense PylRS showed a clear preference toward the homologous tRNAPyl which it charged at a rate 10 times greater than the archaeal tRNAs (Figure 2F).
3.5 The archaeal PylRS amino-terminal domain enables activity in vivo
The robust enzymatic activity of D. hafniense PylRS demonstrates that the amino-terminal domain is dispensable for bacterial PylRS activity in vitro (this work and [6]). Nevertheless, its presence in archaeal PylRSs may be responsible for the significant improvement in archaeal PylRS affinity toward tRNAPyl, suggesting a positive contribution to tRNA binding from this part of the PylRS molecule. In order to assess whether this region is important for aminoacylation in vivo, we truncated the first 106 amino acids of the M. barkeri Fusaro PylRS. This more stringent test of aminoacylation takes into account the competition between non-cognate tRNAs in the cellular context. The functionality of the resulting truncated enzymePylRSΔ1–106 was compared to those of D. hafniense and wild type M. barkeri Fusaro PylRSs as measured by a previously described in vivo suppression assay [12]. In this experiment, PylRS activity is correlated to the level of β-galactosidase generated by read-through of an in-frame amber codon located within the coding sequence of a chromosomally encoded lacI-lacZ fusion reporter system. The E. coli strain XAC/A24 which bears this reporter system, was transformed with plasmid borne copies of D. hafniense and M. barkeri Fusaro (wild type or truncated) pylS genes in conjunction with the plasmid borne M. barkeri Fusaro or D. hafniense pylT genes. The transformants were subsequently grown in LB medium containing 10 mM Cyc.
The E. coli strain bearing the wild type M. barkeri Fusaro pylS generated robust β-galactosidase activity when co-transformed with either M. barkeri Fusaro or D. hafniense pylT (Table 2). These results confirmed our in vitro observation that D. hafniense and M. barkeri Fusaro tRNAPyl are equally good substrates for the wild type M. barkeri Fusaro PylRS. In contrast, neither the naturally truncated D. hafniense PylRS nor the M. barkeri Fusaro PylRSΔ1–106 in which the entire amino-terminal domain was deleted, promoted production of detectable β-galactosidase activity (Table 2). The in vivo charging level of tRNAPyl in these cells was assessed by acid urea gel electrophoresis [22] (Fig. 3). No tRNAPyl aminoacylation was observed in strains expressing D. hafniense PylRS or the amino-terminal truncated M. barkeri Fusaro PylRS (Fig. 3, Table 2). In contrast, good Cyc-tRNAPyl formation was observed in cells carrying the wild-type M. barkeri Fusaro PylRS. This suggests that the truncated enzyme did not productively interact with tRNAPyl in the cellular context when competing noncognate tRNAs are present. This was confirmed by in vivo binding experiments using the yeast three hybrid technique which showed that while the wild type M. barkeri Fusaro PylRS could bind tRNAPyl, such binding was completely abolished upon truncation of the M. barkeri Fusaro amino-terminal domain (data not shown). Taken together our results are consistent with a role for the PylRS amino-terminal domain in tRNAPyl binding.
Table 2. Suppression efficiency of M. barkeri Fusaro PylRS variants.
Suppression efficiency was measured by β-galactosidase activity in E. coli strain XAC/A24. 100% corresponds to 3800 Miller units [13]. In the absence of Cyc, background suppression in all cases was less than 1%. M. barkeri Δ1-NN refers to PylRS N-terminal truncations: Δ1–16 (protein start at M17), Δ1–30 (protein start at M31), Δ1–73 (protein start at M74), Δ1–106 (protein start at M107).
| PylRS | tRNAPyl | Suppression efficiency (%) |
|---|---|---|
| M. barkeri wild type | M. barkeri | 100 |
| D. hafniense | 98 | |
| D. hafniense wild type | M. barkeri | <1 |
| D. hafniense | <1 | |
| M. barkeri Δ1–16 | M. barkeri | 10 |
| M. barkeri Δ1–30 | M. barkeri | <1 |
| M. barkeri Δ1–73 | M. barkeri | <1 |
| M. barkeri Δ1–106 | M. barkeri | <1 |
| M. barkeri D2A | M. barkeri | 5 |
| M. barkeri K3A | M. barkeri | 20 |
| M. barkeri K4A | M. barkeri | 5 |
| M. barkeri D7A | M. barkeri | 95 |
| M. barkerii S11A | M. barkeri | 5 |
| M. barkeri T13A | M. barkeri | 5 |
| M. barkeri W16A | M. barkeri | 65 |
| M. barkeri S18A | M. barkeri | 95 |
| M. barkeri R19A | M. barkeri | 95 |
| M. barkeri G21L | M. barkeri | 95 |
| M. barkeri H24A | M. barkeri | 5 |
| M. barkeri I26G | M. barkeri | 20 |
| M. barkeri D2A/K4A | M. barkeri | <1 |
| M. barkeri S11A/T13A | M. barkeri | <1 |
Fig. 3.
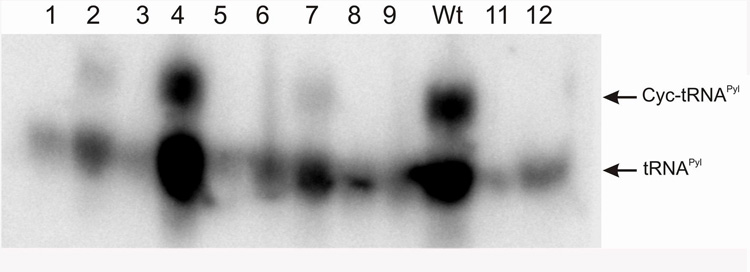
In vivo aminoacylation of tRNAPyl as visualized by northern hybridization. Total tRNA was extracted from E. coli XAC/A24 strain transformed with plasmids bearing M. barkeri Fusaro pylT and either M. barkeri Fusaro PylRS mutants D2A (Lane 1), K3A (Lane 2), K4A (Lane 3), D7A (Lane 4), S11A (Lane 5), T13A (Lane 6), W16A (Lane 7), D2A/K4A (Lane 8), S11A/T13A (Lane 9), wild type M. barkeri Fusaro PylRS (Wt), wild type D. hafniense PylRS (Lane 11) and M. barkeri Fusaro PylRS truncated from its first 106 amino acids (Lane 12). All transformants were grown in the presence of 10mM Cyc. The resulting tRNA products were loaded onto an acid gel. After transfer to a nitrocellulose membrane, the samples were hybridized with a tRNAPyl specific oligonucleotide probe. The positions of tRNAPyl and Cyc-tRNAPyl are indicated.
In order to determine which region of the amino-terminus is important in promoting in vivo activity, we sequentially truncated the amino-terminal domain of the M. barkeri Fusaro PylRS. The position at which the truncations were made was selected based on secondary structure prediction; we respected the integrity of the proposed α-helices and β-sheets. Three amino-terminal truncations of 16, 30 and 73 amino acids (PylRS mutants Δ1–16, Δ1–30 and Δ1–73) were made in M. barkeri Fusaro pylS gene and the resulting enzyme functionality was assayed using the E. coli in vivo suppression method. While the PylRS truncated at positions 30 and 73 were unable to promote UAG suppression, some activity—though modest when compared to the wild type—could be detected when the cells were transformed with the shortest 16 amino acid truncation (Table 2). The in vivo ability of the wild type and truncated PylRSs to bind tRNAPyl was assayed using the yeast three hybrid method. While PylRSs truncated at positions 30 and 73 were unable to form a productive complex with their cognate tRNA, some binding could be recovered with the shortest truncation, although, as in the suppression experiment, to an extent much less than with the wild type PylRS (data not shown).
3.6 Residues from the archaeal PylRS amino-terminal domain affecting activity in vivo
Since the deletion of the first thirty residues significantly reduced in vivo suppression levels, we selected 12 positions in this region for mutagenesis (Table 2). Six of these residues are completely conserved in the PylRS sequences, while the remaining residues were selected based on their ability to engage in potential hydrogen bond interactions with tRNAPyl. We tested the ability of the corresponding mutant proteins to promote in vivo suppression. The K3A and I26G mutations resulted in a moderate 5-fold reduction in PylRS/tRNAPyl mediated UAG suppression. The most significant effects reduced suppression efficiency to 5% or less of the wild type levels, and these included the D2A, K4A, S11A, T13A, and H24A mutants as well as the D2A/K4A and S11A/T13A double mutants. The level of in vivo tRNAPyl aminoacylation was visualized by acid urea gel electrophoresis, and for the mutants assayed the charging levels agreed with the suppression results; Cyc-tRNAPyl was only observed in cells carrying PylRS mutants K3A, D7A and W16A (Fig. 3).
4. Discussion
Compared to the longer Methanosarcinaceae PylRSs the D. hafniense enzyme appears to be at a disadvantage. Since we do not know whether in D. hafniense PylSn and PylRS are both expressed as a non-covalently associated holoenzyme, we attempted to reconstitute this system in E. coli. Expression of PylSn in E. coli leads to a barely soluble protein, the addition of which to purified PylRS did not change the enzyme’s behavior. Co-expression of D. hafniense PylSn and PylRS in E. coli did not yield the desired holoenzyme. While our archaeal PylRS mutant that is most similar to the D. hafniense PylRS (M. barkeri Δ1–106) is active in forming Cyc-tRNAPyl in vitro, it is only 4.3% as efficient as the wild type enzyme.
The D. hafniense enzyme, at least in the absence of PylSn, is only able to efficiently aminoacylate its homologous tRNAPyl in vitro, but not in vivo. These data when taken together with our experiments on PylRS-tRNAPyl binding in addition to our in vitro and in vivo aminoacylation assays of tRNAPyl by amino terminally truncated PylRSs lead to the conclusion that the amino terminal domain is required in the competitive cellular context and that the role of this domain is in tRNA binding and recognition. Consistent with this observation is the fact that the M. barkeri Fusaro PylRS makes contacts with nucleotides in the tRNA anticodon loop [14], while this is not the case for the D. hafniense catalytic core domain that recognizes the discriminator base G73 and the first base pair (G1:C72) of tRNAPyl [6].
We further probed the role of the amino-terminal domain with a series of PylRS mutants. Deletion mutants seem to indicate that the first 30 amino acids of this domain are essential for charging of tRNAPyl in vivo. Specific site directed mutations in this portion of the molecule revealed that D2, K4, S11, T13 and H24 play an important role in the PylRS tRNAPyl interaction. While the yeast three-hybrid data supported the notion that these mutations resulted in lower affinity of PylRS for tRNAPyl, the observed decrease in suppression efficiency could also result from perturbation of the tertiary structure of the protein.
For several reasons (see review [19]), PylRS is a unique enzyme among the tRNA synthetases. Not only does its tRNA recognition domain show no sequence similarity to any other proteins, this domain is present as a split gene in the bacterial examples [5, 15]. While other aaRSs exist as split genes, such as the Aquifex aeolicus LeuRS, in which the two halves of the catalytic core domain are separated [23], or the free-standing editing domain in some crenarchaeal ThrRSs [24, 25], the bacterial PylRSs are the first example of an aaRS that is split between the tRNA recognition domain and the catalytic core.
While we found that the catalytic core domain of the D. hafniense PylRS is not functional in E. coli, the situation could be markedly different in D. hafniense itself. Other aaRS split genes, including the A. aeolicus LeuRS [23] and AlaRS of Nanoarchaeum equitans [26], are expressed to form non-covalently bound, functional holoenzymes. Although the situation may be similar for the D. hafniense PylRS, why any of these genes exist in split forms is unknown. Indeed, the biochemical mystery of how the bacterial Pyl-encoding system functions, namely how or even if the PylRS amino-terminal and catalytic core domains associate in these bacterial examples is a fascinating and still open question. Since our results show that the amino-terminal domain is required for Pyl-tRNAPyl formation in vivo, we find it likely that PylSn has been selectively conserved in D. hafniense and the Olavius endosymbiont during the course of evolution. Future biochemical and genetic studies will be needed to resolve this question.
Supplementary Material
Acknowledgements
We thank Carina Frauer, Darrick Li, Benfang Ruan and Kelly Sheppard for their assistance; Richard Villemur, William Metcalf and Kevin Hill for the gift of D. hafniense, M. acetivorans and M. thermophila genomic DNAs. We thank Nikos Kyrpides for the access to the Olavius algarvensis community meta-genomic sequences. This work was supported by grants from the National Institute of General Medical Sciences, the Department of Energy, and the National Science Foundation.
Abbreviations
- aaRS
aminoacyl-tRNA synthetase
- Pyl
pyrrolysine
- PylRS
pyrrolysyl-tRNA synthetase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blight SK, Larue RC, Mahapatra A, Longstaff DG, Chang E, Zhao G, Kang PT, Green-Church KB, Chan MK, Krzycki JA. Direct charging of tRNACUA with pyrrolysine in vitro and in vivo. Nature. 2004;431:333–335. doi: 10.1038/nature02895. [DOI] [PubMed] [Google Scholar]
- 2.Polycarpo C, Ambrogelly A, Bérubé A, Winbush SM, McCloskey JA, Crain PF, Wood JL, Söll D. An aminoacyl-tRNA synthetase that specifically activates pyrrolysine. Proc. Natl. Acad. Sci. USA. 2004;101:12450–12454. doi: 10.1073/pnas.0405362101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soares JA, Zhang L, Pitsch RL, Kleinholz NM, Jones RB, Wolff JJ, Amster J, Green-Church KB, Krzycki JA. The residue mass of L-pyrrolysine in three distinct methylamine methyltransferases. J. Biol. Chem. 2005;280:36962–36969. doi: 10.1074/jbc.M506402200. [DOI] [PubMed] [Google Scholar]
- 4.Longstaff DG, Blight SK, Zhang L, Green-Church KB, Krzycki JA. In vivo contextual requirements for UAG translation as pyrrolysine. Mol. Microbiol. 2007;63:229–241. doi: 10.1111/j.1365-2958.2006.05500.x. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan G, James CM, Krzycki JA. Pyrrolysine encoded by UAG in Archaea: charging of a UAG-decoding specialized tRNA. Science. 2002;296:1459–14562. doi: 10.1126/science.1069588. [DOI] [PubMed] [Google Scholar]
- 6.Herring S, Ambrogelly A, Polycarpo CR, Söll D. Recognition of pyrrolysine tRNA by the Desulfitobacterium hafniense pyrrolysyl-tRNA synthetase. Nucleic Acids Res. 2007;35:1270–1278. doi: 10.1093/nar/gkl1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987;15:8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen JD, Parsons SM. Nitrocellulose filter binding: quantification of the histidyl-tRNA-ATP phosphoribosyltransferase complex. Anal. Biochem. 1979;92:22–30. doi: 10.1016/0003-2697(79)90620-1. [DOI] [PubMed] [Google Scholar]
- 9.Bovee ML, Yan W, Sproat BS, Francklyn CS. tRNA discrimination at the binding step by a class II aminoacyl-tRNA synthetase. Biochemistry. 1999;38:13725–13735. doi: 10.1021/bi991182g. [DOI] [PubMed] [Google Scholar]
- 10.Ambrogelly A, Korencic D, Ibba M. Functional annotation of class I lysyl-tRNA synthetase phylogeny indicates a limited role for gene transfer. J. Bacteriol. 2002;184:4594–4600. doi: 10.1128/JB.184.16.4594-4600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfson AD, Uhlenbeck OC. Modulation of tRNAAla identity by inorganic pyrophosphatase. Proc. Natl. Acad. Sci. USA. 2002;99:5965–59670. doi: 10.1073/pnas.092152799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polycarpo C, Herring S, Bérubé A, Wood JL, Söll D, Ambrogelly A. Pyrrolysine analogues as substrates for pyrrolysyl-tRNA synthetase. FEBS Lett. 2006;580:6695–6700. doi: 10.1016/j.febslet.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY; 1972. [Google Scholar]
- 14.Ambrogelly A, Gundllapalli S, Herring S, Polycarpo C, Frauer C, Söll D. Pyrrolysine is not hardwired for cotranslational insertion at UAG codons. Proc. Natl. Acad. Sci. USA. 2007;104:3141–3146. doi: 10.1073/pnas.0611634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woyke T, Teeling H, Ivanova NN, Huntemann M, Richter M, Gloeckner FO, Boffelli D, Anderson IJ, Barry KW, Shapiro HJ, Szeto E, Kyrpides NC, Mussmann M, Amann R, Bergin C, Ruehland C, Rubin EM, Dubilier N. Symbiosis insights through metagenomic analysis of a microbial consortium. Nature. 2006;443:950–955. doi: 10.1038/nature05192. [DOI] [PubMed] [Google Scholar]
- 16.Ferry JG. Enzymology of the fermentation of acetate to methane by Methanosarcina thermophila. Biofactors. 1997;6:25–35. doi: 10.1002/biof.5520060104. [DOI] [PubMed] [Google Scholar]
- 17.Longstaff DG, Larue RC, Faust JE, Mahapatra A, Zhang L, Green-Church KB, Krzycki JA. A natural genetic code expansion cassette enables transmissible biosynthesis and genetic encoding of pyrrolysine. Proc. Natl. Acad. Sci. USA. 2007;104:1021–1026. doi: 10.1073/pnas.0610294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao B, Zhao G, Kang PT, Soares JA, Ferguson TK, Gallucci J, Krzycki JA, Chan MK. Reactivity and chemical synthesis of L-pyrrolysine- the 22nd genetically encoded amino acid. Chem. Biol. 2004;11:1317–13124. doi: 10.1016/j.chembiol.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Ambrogelly A, Palioura S, Söll D. Natural expansion of the genetic code. Nat. Chem. Biol. 2007;3:29–35. doi: 10.1038/nchembio847. [DOI] [PubMed] [Google Scholar]
- 20.Sprinzl M, Horn C, Brown M, Ioudovitch A, Steinberg S. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1998;26:148–153. doi: 10.1093/nar/26.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hook B, Bernstein D, Zhang B, Wickens M. RNA-protein interactions in the yeast three-hybrid system: Affinity, sensitivity, and enhanced library screening. RNA. 2005;11:227–233. doi: 10.1261/rna.7202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varshney U, Lee CP, RajBhandary UL. Direct analysis of aminoacylation levels of RNAs in vivo. Application to studying recognition of Escherichia coli initiator tRNA mutants by glutaminyl-tRNA synthetase. J. Biol. Chem. 1991;266:24712–24718. [PubMed] [Google Scholar]
- 23.Gouda M, Yokogawa T, Asahara H, Nishikawa K. Leucyl-tRNA synthetase from the extreme thermophile Aquifex aeolicus has a heterodimeric quaternary structure. FEBS Lett. 2002;518:139–143. doi: 10.1016/s0014-5793(02)02675-3. [DOI] [PubMed] [Google Scholar]
- 24.Woese CR, Olsen GJ, Ibba M, Söll D. Aminoacyl-tRNA synthetases, the genetic code, and the evolutionary process. Microbiol. Mol. Biol. Rev. 2000;64:202–236. doi: 10.1128/mmbr.64.1.202-236.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korencic D, Ahel I, Schelert J, Sacher M, Ruan B, Stathopoulos C, Blum P, Ibba M, Söll D. A freestanding proofreading domain is required for protein synthesis quality control in Archaea. Proc. Natl. Acad. Sci. USA. 2004;101:10260–10265. doi: 10.1073/pnas.0403926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waters E, Hohn MJ, Ahel I, Graham DE, Adams MD, Barnstead M, Beeson KY, Bibbs L, Bolanos R, Keller M, Kretz K, Lin X, Mathur E, Ni J, Podar M, Richardson T, Sutton GG, Simon M, Söll D, Stetter KO, Short JM, Noordewier M. The genome of Nanoarchaeum equitans: insights into early archaeal evolution and derived parasitism. Proc. Natl. Acad. Sci. USA. 2003;100:12984–12988. doi: 10.1073/pnas.1735403100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


