Abstract
Purpose
To introduce a four dimensional tomotherapy treatment technique with improved motion control and patient tolerance.
Material and Methods
CT images at ten breathing phases were acquired for treatment planning. The full exhalation phase was chosen as planning phase and the CT images at this phase were used as treatment planning images. ROI delineation was the same as traditional treatment planning except that no breathing motion margin was used in CTV-PTV expansion. The correlation between delivery and breathing phases was set assuming a constant gantry speed and a fixed breathing period. Deformable image registration yielded the deformation fields at each phase relative to the planning phase. With the delivery/breathing phase correlation and voxel displacements at each breathing phase, a 4D tomotherapy plan was obtained by incorporating the motion into inverse treatment plan optimization. A combined laser/spirometer breathing tracking system has been developed to monitor patient’s breathing. This system is able to produce stable and reproducible breathing signals representing tidal volume.
Results
We compared 4D tomotherapy treatment planning method to conventional tomotherapy on static target. The results showed that 4D tomotherapy can achieve similar dose distributions on a moving target as conventional delivery on a stationary target. Regular breathing motion is fully compensated by motion-incorporated BSD planning. 4D tomotherapy also has close to 100% duty cycle and does not prolong treatment time.
Conclusion
Breathing synchronized delivery is a feasible 4D tomotherapy treatment technique with improved motion control and patient tolerance.
Keywords: Tomotherapy, breathing motion, respiratory gating, deformable image registration, treatment plan optimization
INTRODUCTION
Helical tomotherapy (1,2), designed for image guided radiotherapy, is one form of intensity modulated radiation therapy (IMRT) that is delivered in continuous helix. Its fan shaped beam is modulated by a binary multi-leaf collimator (MLC) that is able to open and close its leaves within 40ms. Online megavoltage CT (MVCT) images (3) may be acquired prior to treatment for patient setup and dose reconstruction (4). Helical tomotherapy is commercially available by TomoTherapy (TomoTherapy Inc., Madison, WI).
Radiotherapy plays an important role in non-small-cell lung cancer (NSCLC) treatment. Despite recent advances, 5-year survival rate remains low at only 5% to 10% (5,6). Local control with the standard 60-Gy is still low. Recent studies by Martel et al show dose escalation improves local control rate (7). Doses exceeding 100-Gy are currently being evaluated. However, the maximum dose that can be safely delivered is limited by the tolerance of the adjacent normal lung toxicity. Tomotherapy potentially is a useful tool for dose escalation in lung cancer treatments. A high dose gradient provided by tomotherapy is able to spare more normal tissue from intensive dose prescribed to the target. Rotating dose delivery spreads the entrance and exit doses to a larger volume. Biological equivalent dose (BED) increases faster than physical dose. Thereby dose spreading may reduce normal tissue complication. Treatment planning studies by Scrimger et al demonstrated that the dose to normal lung, whether measured by NTDmean or V20, was reduced by tomotherapy and dose escalation could be as high as 160-Gy in certain patients (8). The cost of spreading dose to a larger volume is the decrease of volume with zero dose. Although there is concern about dose spreading, lung cancer radiation treatment by tomotherapy has been widely employed in many institutions.
Respiratory motion of the lung targets can be as large as 1.5~2cm. Normal tissue included in the planning target volume (PTV) in lung cancer treatment may be very large. Although larger beam penumbra due to low density of lung tissue may alleviate geometric miss in some degree, a motion control or compensation method is desired to reduce the PTV margin further, especially in hypofraction treatments in which large amount doses are delivered in fewer fractions.
Current motion control techniques include free breathing gating (9-11), breath-hold gating (12-14) and target tracking (15-17). Conventional techniques are not easily implemented on tomotherapy due to its rotating delivery mode. To perform free breathing gating on tomotherapy, patients have to breathe with a periodic breathing pattern; Similarly, patients have to be able to hold and breathe with fixed pre-planned durations in breath-hold gating, which may be very difficult for the lung patients. The binary MLC is not able to track motion in craniocaudal direction. Principally it is possible to track the motion using independent jaws if the motion is primarily in craniocaudal direction (18).
A new technique with better patient tolerance and motion control is desired for tomotherapy. For this purpose, Breathing Synchronized Delivery (BSD) is developed for lung cancer tomotherapy treatment. During BSD treatment, patient breathes following a visually displayed guiding cycles. Target motion and deformation are included into the treatment plan optimization (19).
METHODS AND MATERIALS
Breathing tracking and visual guidance
Abdominal displacement measurement with laser or camera has been widely used in free breathing phase gating. 4D radiotherapy such as the BSD technique has 100% duty cycle. Scaling error from daily setup variation may cause tracking uncertainty. Tidal volume measurement by spirometer has better correlation with target position. However, the signal drift may confuse the patient during 4D treatments (20). To improve tracking accuracy, a laser/spirometer combined tracking system has been developed (Figure 1). In this system, a spirometer (Medical Graphics Cooperation, St Paul, Minnesota) and a laser displacement sensor (LAP Laser, Boca Raton, Florida) are both used to monitor patient’s breathing. The displacement measurement by the laser is converted into tidal volume by using a scaling factor obtained from the correlation between spirometer and laser measurements. During treatment, the relatively position between the laser to the patient does not change. So once the correlation is determined, only laser measurement is necessary in the subsequent breathing monitoring.
Figure 1.
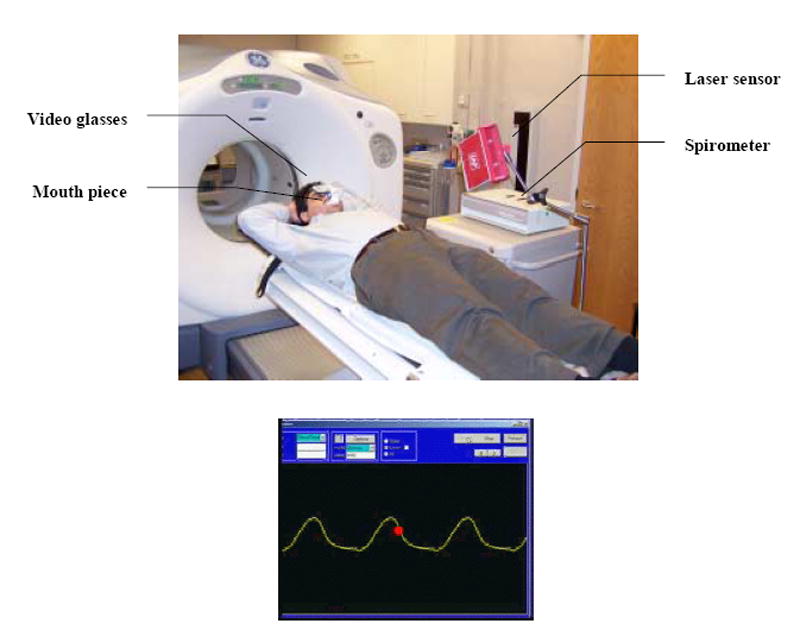
A laser/spirometer combined respiratory breathing tracking system for breathing tracking (top) and the computer interface (bottom). Computer screen is displayed to the patient via video glasses. The breathing guide moves from right to left with constant speed and the breathing phase indicator (red ball) moves up and down according to instantaneous breathing phases.
A computer software has been developed for the purpose of breathing tracking. One of the patient’s free breathing cycles is chosen as guiding cycle. This patient specific guiding cycle is used as reference breathing pattern in both 4D-CT scan and tomotherapy treatments. A breathing guide is obtained by repeating the guiding cycle. The breathing guide moves from right to left on computer screen (Figure 1). Patient’s breathing indicator, shown as a ball, moves up and down in the middle of the screen. We used video glasses to display the computer screen to the patient. The patient controls his/her breathing to match the breathing indicator on the moving breathing guide. A regular and reproducible breathing pattern is obtained.
4D lung image sequence acquisition
Dynamic CT (4D-CT) scanned each table position over a full breathing period in cine mode (21, 22). The images were then binned into different breathing phases in a post-processing procedure using commercial software package (4D-Advantage, GE medical system). Scanning protocol was 140 kVp, 100mAs, 0.5 second gantry rotation, cine mode with 4.8 second duration (based on patient’s breathing period). Using GE LightSpeed scanner with 4 row detector, 1 cm range was covered in each table position with 2.5 mm slice thickness. The whole acquisition time was less than 5 minutes. The images were then binned into 10 equally separated phases.
During the 4D scans, patients breathed following the breathing guide via visual guidance. Such an approach not only can reduce binning artifact from irregular breathing, it also guarantees that the motion pattern in 4D images actually represents the motion pattern in treatment. Clinical trial protocol was approved by institutional review board (IRB).
Region of Interest (ROI) Delineation
Irregular breathing pattern causes binning artifacts. It is noted that the artifacts are minimum at full exhalation phase. Thus, the exhalation phase was selected as the planning phase. Current tomotherapy treatment planning system (TPS) does not have contouring functions. Contouring of the ROIs on the planning phase was performed by using TPS Pinnacle™ (Philips Radiation Oncology, Madison, WI). ROI delineation is the same as traditional lung cancer treatment except that no breathing motion margin is added in CTV-PTV expansion. The contours and the images at all phases were then imported to TomoTherapy BSD optimizer prototype.
Deformable image registration and validation
Various deformable image registration algorithms are available for deformable image registration. A deformable image registration tool based on calculus of variations (23) is implemented in TomoTherapy’s BSD package. The CT images at planning phase were used as reference images and the images at all other phases were used as floating image. Deformable image registration transforms the floating images to maximize the similarity between the floating images and reference images. Deformable image registration generates a displacement vector maps by tracking the displacement of each voxel. The result of deformable image registration was validated by visually inspecting the difference between the reference images and transformed floating images.
Correlating delivery and breathing phase
A guiding cycle was picked from the patient’s own breathing curve. The guiding cycle was repeated to construct a guiding breathing curve. Tomotherapy gantry rotates with a fixed speed. Delivery phases represented by projection number were correlated to the breathing phases prior to treatment planning. With this correlation, the deformation state of the lung was set in treatment planning. This correlation can be reproduced by instructing the patient to track the breathing guide during treatments. For this patient, the breathing (guiding cycle) period is 4.1 second. We have set gantry speed as 20 second per rotation.
Motion incorporated treatment plan optimization
4D CT images, breathing/delivery phase correlation and deformation field at each phase were used in 4D tomotherapy treatment plan optimization. From user’s point of view, 4D tomotherapy treatment planning is the same as conventional tomotherapy planning. The detailed procedure of motion incorporated treatment plan optimization has been shown in previous publication (18). Here we briefly summarize the procedure in 4D tomotherapy optimization (Figure 2):
Figure 2.
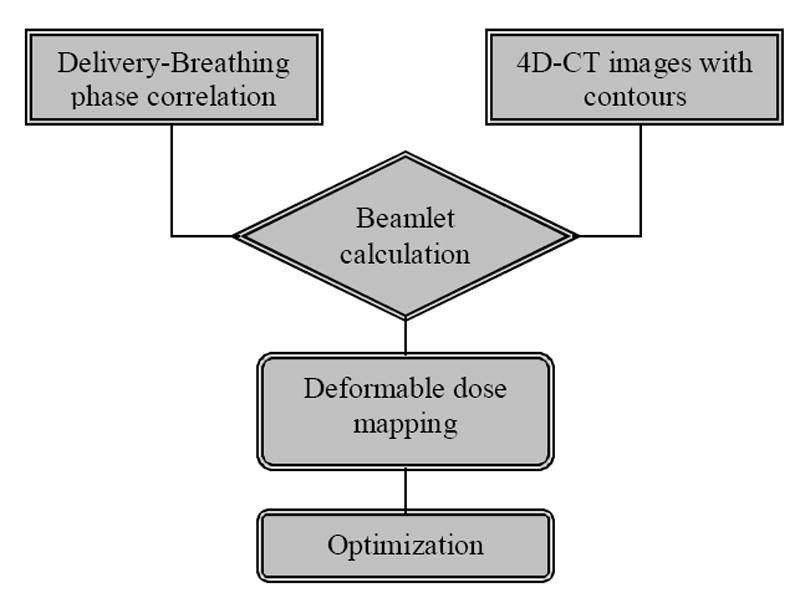
Flow chart of tomotherapy BSD treatment plan optimization (19). BSD tomotherapy treatment planning uses a priori knowledge of the breathing/delivery phase correlation to incorporate the breathing motion into optimization.
With the knowledge of the breathing/delivery phase correlation, beamlets at a specific delivery phase are calculated by using the CT images at corresponding breathing phase.
With the displacement maps from deformable image registration, beamlet dose distributions are then mapped back to the planning phase.
In treatment plan optimization, the motion is included in the optimization by using the deformed beamlets.
BSD tomotherapy planning is based on a priori knowledge of breathing/delivery phase correlation. This correlation must be reproduced during treatment.
BSD tomotherapy delivery
During treatment, the patients will be coached to breathe following the breathing guide. Treatment delivery starts when the patient’s breathing is synchronized with the guiding cycle. Patient’s breathing is monitored with a respiratory motion tracking system, such as the spirometer/laser combined system. In order to synchronize breathing with delivery, the treatment machine should provide treatment progress (i.e. projection number) information to breathing monitoring interface so that the breathing guide moves in concert with treatment delivery. BSD tomotherapy technique is still in development stage. Up to date, no patient has yet been treated using this technique.
RESULTS
The laser/spirometer combined system tested on a healthy volunteer. Figure 3 shows the correlation between spirometer and laser signals. The curves moved downward due to the drift of spirometer signal. The correlation was predominantly linear and the average slope K was about 0.458 l/cm. The laser signals were converted into tidal volume reading with the correlation factor K. The signals before and after conversion are also shown in Figure 3. The laser signals were similar to the spirometer reading after conversion.
Figure 3.
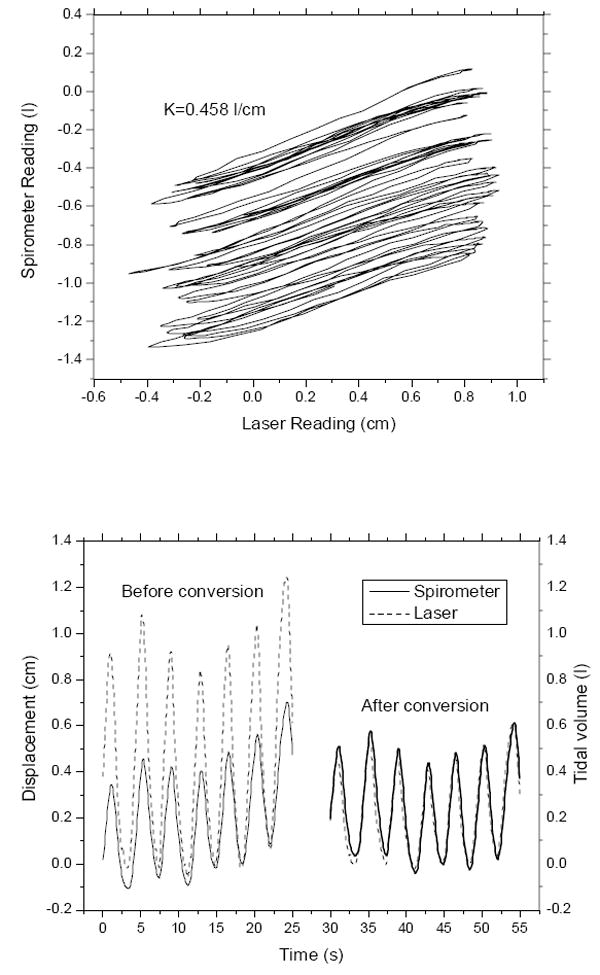
Calibration of laser displacement signal into tidal volume by a spirometer. Top: the correlation between laser and spirometer signals for a specific patient setup. Bottom: The laser signal before and after calibration. The correlation between laser and spirometer is approximately linear (K is the slope). After conversion, the laser signals match spirometer tidal volume reading.
4D-CT images were acquired using a 4-row detector LightSpeed™ CT scanner (GE Medical System, Waukesha, WI). Figure 4 shows the images at two selected phases. For this particular patient, target motion was the largest in craniocaudal direction with a magnitude of about 1.5 cm. Owning to irregular breathing during the scan, some artifacts was noticeable in the binned images. Relatively, the artifact was most insignificant at the exhalation phase.
Figure 4.
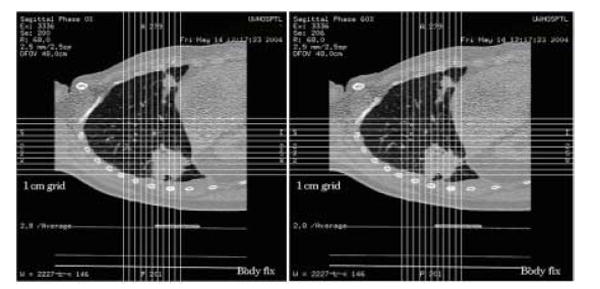
CT images at full inhalation phase (left) and full exhalation phase (right). 4D-CT images were acquired by GE LightSpeed CT/PET scanner with 4 row detectors. The images were binned into different breathing phases by 4D Advantage package provided by GE.
Deformable image registration was performed using an algorithm implemented in TomoTherapy’s BSD package (23). The algorithm was robust and fast when we tested on 4D-CT images. The images at planning phase were chosen as reference images; Images at all other phases were chosen as floating images. Deformable image registration transforms the floating images into the reference images to maximize their similarity. Figure 5 shows the lung images before and after image registration. After image registration the two images were almost identical.
Figure 5.
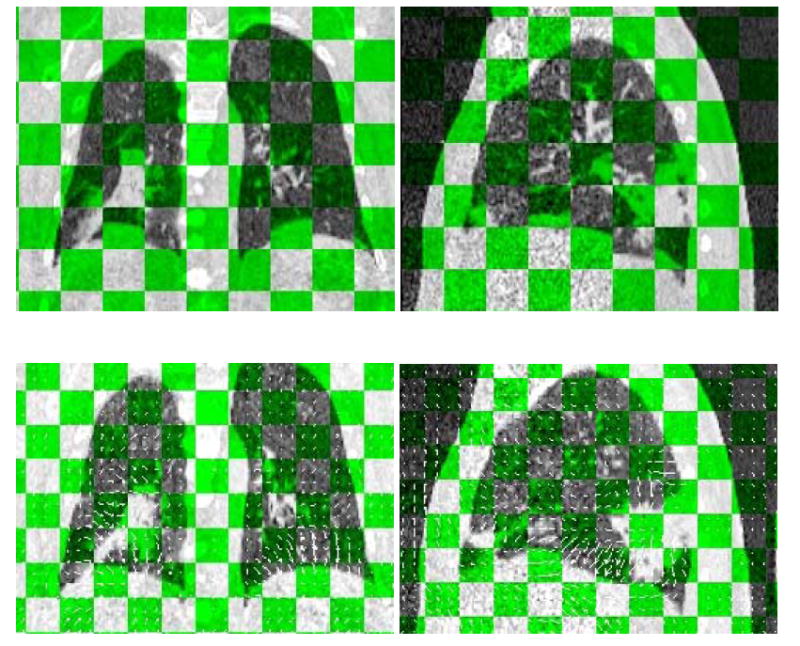
CT images before (up) and after (down) deformable image registration. Displacement vector fields are also shown in images after registration. Tilted view shows the floating images and reference images alternatively.
Prior to treatment planning, the breathing and delivery phase correlation was determined. The breathing period of this particular patient is 4.1 second. Gantry rotation speed was set as 20 second per rotation. Each rotation included 51 projections. Treatment started at breathing phase #0. With constant gantry rotation speed and breathing rate, the following breathing/delivery phase correlation was determined and shown in Figure 6.
Figure 6.
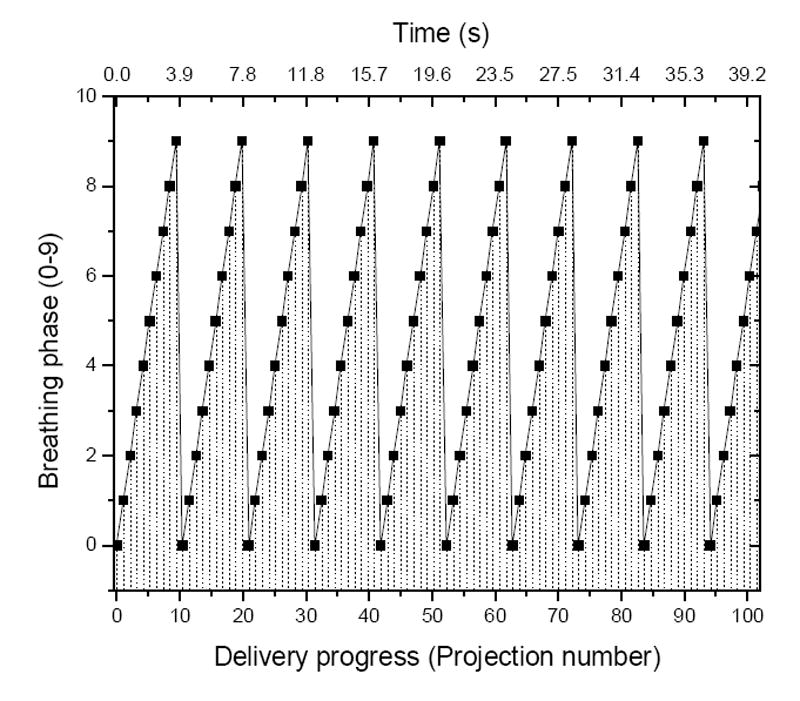
Breathing and delivery phase correlation. Patient specific breathing period is 4.1 s, gantry rotation speed is 20 s per rotation. Each rotation includes 51 projections. The first projection starts in breathing phase #0.
BSD treatment planning was performed on the by TomoTherapy 4D optimizer prototype. 4D CT images, displacement maps and breathing/delivery phase correlation were the inputs for 4D tomotherapy planning. The correspondence between breathing phases and delivery projections were predetermined and shown in Figure 6. Beamlet dose distributions were calculated using the CT images at corresponding breathing phases. With displacement maps at corresponding breathing phases, beamlets were then mapped back to the primary phase through deformable dose mapping. Most beamlets were deformed after dose mapping due to the deformation of the lung. Beamlet-based optimization incorporates target motion by using the deformed beamlets. From user’s point-of-view, 4D optimizer is the same as conventional optimizer. All the procedures were performed automatically without user intervention.
Tomotherapy treatment plan is a sinogram with beamlet intensities of each beam in each projection. Figure 7 shows the treatment plan generated by 4D tomotherapy planning. Take a closer look at the sinogram, we noticed that at beginning and ending of treatment, the intensities became discontinuous. This is because the targets move in and out of the treatment plane. Thus, BSD tomotherapy is not exact but very close to 100% duty cycle. In order to compare dosimetry results, another conventional tomotherapy treatment planning was performed on the static images at the planning phase. This assumes the tumors do not move during the treatment. Dose volume histograms (DVH) of the BSD plan and static plan were plotted in Figure 8. Although the static plan looks a little better in sparing the lung and spinal cord, the BSD plan have better coverage in PTV. The little difference may come from the slightly difference in optimization parameters. In principle, BSD plan should have the similar results as the static plan. This is more clearly shown by a 2D case in previous publication (19).
Figure 7.

The BSD tomotherapy treatment plan. The two curves indicate there were two targets at two different positions. At beginning and ending, beam intensities became discontinuous because the targets moved in and out of the treatment plane.
Figure 8.
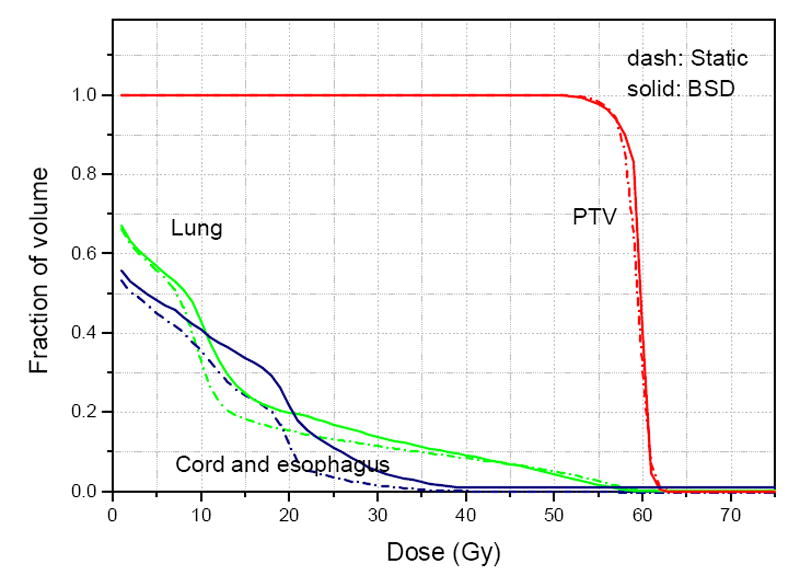
DVH curves of the treatment planning for breathing synchronized delivery (BSD) and conventional static delivery. Similar dose coverage on PTV and lung sparing were obtained. The little difference may come from different optimization parameters.
DISCUSSION
The respiratory motion blurring within this period can be neglected. Although beamlets are deformed, the dose gradients are still preserved. In addition to that, the number of beamlets that have contributions on the mobile targets is about the same as static delivery. Thus, as we observed from Figure 8, compared with conventional static treatment planning, BSD planning achieves similar dose distribution on a mobile target as conventional planning on static target.
In this paper, we used a lung cancer case as an example to illustrate the BSD method. In principle, BSD may also be applied to other sites to reduce respiratory motion effects, such as liver or breast. BSD method cannot compensate cardiac motion since cardiac motion is not controllable. Moreover, with a maximum duration about 0.4 second, each beamlet is significantly blurred by the cardiac motion. Addition motion margin has to be allowed for cardiac motion for BSD planning.
BSD planning performs dose calculation on 4D-CT images. It fully utilizes the information provided by 4D-CT. Complex motion patterns, such as rotation, deformation, hysteresis, have been observed on the targets in the lung. Dynamic CT scan allows patient to breathe freely and such information was recorded in the CT image sequences. Thereby, any complex motion patterns, as long as recorded by 4D-CT and recovered by deformable image registration, are included in BSD treatment planning. In principle, BSD technique does not impose any assumption in target motion pattern.
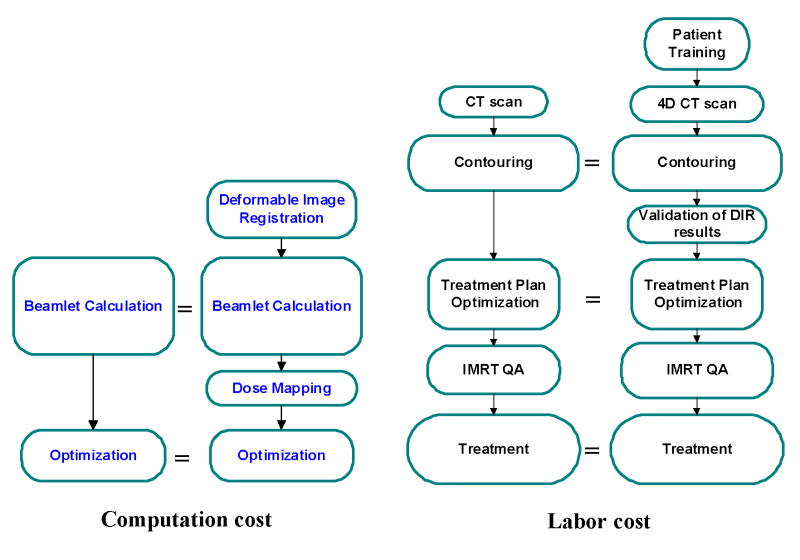
Figure 9 compares the computational and labor costs of BSD tomotherapy with traditional tomotherapy procedures. For computational cost, beamlet calculation is the most computationally expensive procedure in tomotherapy treatment planning. Usually beamlet calculation takes several hours by a 32-CPU computer cluster. The number of beamlets needed to be calculated is approximately the same for BSD as that of conventional static treatment planning. Additional CPU time needed for image registration and dose mapping is insignificant compared with beamlet calculation. Thereby BSD treatment planning does not increase computational cost much. For physicians, ROI delineation is performed on the planning phase CT image only. Additional human interaction is only needed for the validation of deformable image registration results. The deformable image registration tool provided by TomoTherapy’s BSD prototype is robust enough that no human interaction is needed for the first a few cases we have tried. BSD treatment time is not prolonged compared to traditional tomotherapy since it is a 4D technique with near 100% duty cycle.
Figure 9.
Comparisons of BSD tomotherapy with conventional tomotherapy in computation cost and labor cost. The size of boxes approximately represents the workload of each item. BSD treatment is not significantly complicated than traditional tomotherapy.
The major assumption of BSD technique is the reproducibility of breathing/delivery phase correlation. Our initial experience showed that BSD is well tolerated by most of the patients. Other institutions also reported patient can be trained to breathe with a regular breathing pattern (24, 25). In clinical implementation, appropriate training is necessary to improve the tracking performance. During actual BSD treatment, if the deviation of the breathing pattern and breathing guide falls outside a certain threshold, the couth feeding and radiation should be paused. Tomotherapy treatment resumes at the same gantry position after breathing synchronization is regained. A special feedback-control function would be necessary to hold table movement and treatment beams.
Acknowledgments
This work was partially supported by a grant from the National Institute of Health (Award Number P01 CA-88960) and a contract with TomoTherapy Inc. LAP Laser LLC has provided laser displacement sensor for this study.
Footnotes
*Partially presented in Astro annual conference 2003 and AAPM annual conference 2004
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mackie TR, Holmes T, Swerdloff S, et al. Tomotherapy: A new concept for the delivery of dynamic conformal radiotherapy. Med Phys. 1993;20:1709–1719. doi: 10.1118/1.596958. [DOI] [PubMed] [Google Scholar]
- 2.Welsh JS, Patel RR, Ritter MA, et al. Helical tomotherapy: an innovative technology and approach to radiation therapy. Technol Cancer Res Treat. 2002;1:311–316. doi: 10.1177/153303460200100413. [DOI] [PubMed] [Google Scholar]
- 3.Ruchala KJ, Olivera GH, Schloesser EA, et al. Megavoltage CT on a tomotherapy system. Phys Med Biol. 1999;44(10):2597–2621. doi: 10.1088/0031-9155/44/10/316. [DOI] [PubMed] [Google Scholar]
- 4.Kapatoes JM, Olivera GH, Ruchala KJ, et al. A feasible method for clinical delivery verification and dose reconstruction in tomotherapy. Med Phys. 2001;28:528–542. doi: 10.1118/1.1352579. [DOI] [PubMed] [Google Scholar]
- 5.Dillman R, Herndon J, Seagren S. Improved survival in stage III non-small cell lung cancer: seven-year follow up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88:1210–1215. doi: 10.1093/jnci/88.17.1210. [DOI] [PubMed] [Google Scholar]
- 6.Saunders M, Dische S, Barrett A. Randomized multicentre trials of CHART vs conventional radiotherapy in head and neck and non-small cell lung cancer: an interim report. Br J Cancer. 1996;73:1455–1462. doi: 10.1038/bjc.1996.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martel MK, Ten Haken RK, Hazuka MB. Estimation of tumor control probability parameters from 3D dose distribution of non-small cell lung cancer patients. Lung Cancer. 1999;24:31–37. doi: 10.1016/s0169-5002(99)00019-7. [DOI] [PubMed] [Google Scholar]
- 8.Scrimger RA, Tomé WA, Olivera GH, et al. Reduction in radiation dose to lung and other normal tissues using helical tomotherapy to treat lung cancer, in comparison to conventional field arrangements. Am J Clin Onco. 2003;26:70–78. doi: 10.1097/00000421-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Tada T, Minakuchi K, Fujioka T, et al. Lung cancer: intermittent irradiation synchronized with respiratory motion – results of a pilot study. Radiology. 1998;207:779–783. doi: 10.1148/radiology.207.3.9609904. [DOI] [PubMed] [Google Scholar]
- 10.Kubo HD, Len PM, Minohara S, et al. Breathing synchronized radiotherapy program at the University of California Davis Cancer Center. Med Phys. 2000;27:346–353. doi: 10.1118/1.598837. [DOI] [PubMed] [Google Scholar]
- 11.Wagman R, Yorke E, Ford E, et al. Respiratory gating for liver tumors: use in dose escalation. Int J Radiat Oncol Biol Phys. 2003;55:659–668. doi: 10.1016/s0360-3016(02)03941-x. [DOI] [PubMed] [Google Scholar]
- 12.Hanley J, Debois MM, Mah D, et al. Deep inspiration breath-hold technique for lung tumors: the potential value of target immobilization and reduced lung density in dose escalation. Int J Radiat Oncol Biol Phys. 1999;45:603–611. doi: 10.1016/s0360-3016(99)00154-6. [DOI] [PubMed] [Google Scholar]
- 13.Wong JW, Sharpe MB, Jaffray DA, et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys. 1999;44:911–919. doi: 10.1016/s0360-3016(99)00056-5. [DOI] [PubMed] [Google Scholar]
- 14.Dawson LA, Brock KK, Kazanjian S, et al. The reproducibility of organ position using active breathing control (ABC) during liver radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:1410–1421. doi: 10.1016/s0360-3016(01)02653-0. [DOI] [PubMed] [Google Scholar]
- 15.Neicu T, Shirato H, Seppenwoolde U, et al. Synchronized moving aperture radiation therapy (SMART): average tumour trajectory for lung patients. Phys Med Biol. 2003;48:587–598. doi: 10.1088/0031-9155/48/5/303. [DOI] [PubMed] [Google Scholar]
- 16.Keall PJ, Kini VR, Vedam SS, et al. Motion adaptive x-ray therapy: a feasibility study. Phys Med Biol. 2001;46:1–10. doi: 10.1088/0031-9155/46/1/301. [DOI] [PubMed] [Google Scholar]
- 17.Papiez L, Rangaraj D, Keall P. Real-time DMLC IMRT delivery for mobile and deforming targets. Med Phys. 2005;32:3037–3048. doi: 10.1118/1.1987967. [DOI] [PubMed] [Google Scholar]
- 18.Fenwick John. Personal communication. 2003 [Google Scholar]
- 19.Zhang T, Jeraj R, Keller H, et al. Treatment plan optimization incorporating motion. Med Phys. 2004;31(6):1576–1586. doi: 10.1118/1.1739672. [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Keller H, O’Brien MJ, et al. Application of the spirometer in respiratory gated radiotherapy. Med Phys. 2003;30(12):3165–3171. doi: 10.1118/1.1625439. [DOI] [PubMed] [Google Scholar]
- 21.Vedam SS, Keall PJ, Kini VR, et al. Acquiring a four-dimensional computed tomography dataset using an external respiratory signal. Phys Med Biol. 2003;48:45–62. doi: 10.1088/0031-9155/48/1/304. [DOI] [PubMed] [Google Scholar]
- 22.Low DA, Nystrom M, Kalinin E, et al. A method for the reconstruction of four-dimensional synchronized CT scans acquired during free breathing. Med Phys. 2003;30:1254–1263. doi: 10.1118/1.1576230. [DOI] [PubMed] [Google Scholar]
- 23.Lu W, Chen ML, Olivera GH, et al. Fast free-form deformable registration via calculus of variations. Phys Med Biol. 2004;49(14):3067–3087. doi: 10.1088/0031-9155/49/14/003. [DOI] [PubMed] [Google Scholar]
- 24.Neicu T, Berbeco R, Wolfgang J, Jiang SB. Synchronized moving aperture radiation therapy (SMART): improvement of breathing pattern reproducibility using respiratory coaching. Phys Med Biol. 2006;51(3):617–636. doi: 10.1088/0031-9155/51/3/010. [DOI] [PubMed] [Google Scholar]
- 25.George R, Vedam S, Chung T, Ramakrishnan V, Keall PJ. The application of the sinusoidal model to lung cancer patient respiratory motion. Med Phys. 2005;32(9):2850–2861. doi: 10.1118/1.2001220. [DOI] [PubMed] [Google Scholar]