Abstract
During craniofacial development, Meckel’s cartilage and the mandible bone derive from the first branchial arch, and their development depends upon the contribution of cranial neural crest (CNC) cells. We previously demonstrated that conditional inactivation of Tgfbr2 in the neural crest of mice (Tgfbr2fl/fl;Wnt1-Cre) results in severe defects in mandibular development, although the specific cellular and molecular mechanisms by which TGF-β signaling regulates the fate of CNC cells during mandibular development remain unknown. We show here that loss of Tgfbr2 does not affect the migration of CNC cells during mandibular development. TGF-β signaling is specifically required for cell proliferation in Meckel’s cartilage and the mandibular anlagen and for the formation of the coronoid, condyle and angular processes. TGF-β-mediated connective tissue growth factor (CTGF) signaling is critical for CNC cell proliferation. Exogenous CTGF rescues the cell proliferation defect in Meckel’s cartilage of Tgfbr2fl/fl;Wnt1-Cre mutants, demonstrating the biological significance of this signaling cascade in chondrogenesis during mandibular development. Furthermore, TGF-β signaling controls Msx1 expression to regulate mandibular osteogenesis as Msx1 expression is significantly reduced in Tgfbr2fl/fl;Wnt1-Cre mutants. Collectively, our data suggest that there are differential signal cascades in response to TGF–β to control chondrogenesis and osteogenesis during mandibular development.
Keywords: cranial neural crest (CNC), cell proliferation, differentiation, mandible and Meckel’s cartilage development, CTGF, Msx1, TGF-β
Introduction
The mandible is an important structure involved in the essential functions of breathing and mastication. Many human syndromes with craniofacial anomalies include defects in mandible formation. Mandibular development depends on the processes of chondrogenesis and osteogenesis. Specifically, chondrogenesis involves the formation of Meckel’s cartilage and coronoid, condylar and angular cartilage. Mandible bone is formed by intramembranous ossification. To date, little is known about the regulation of chondrogenesis and osteogenesis during mandibular development.
Meckel’s cartilage appears transiently during the early embryonic stage. Previous studies have suggested that the role of Meckel’s cartilage is to serve as a template for mandible formation and also to contribute as part of the mandible bone after ossification (Carda et al., 2005; Melnick et al., 2005; Ramaesh and Bard, 2003). Meckel's cartilage, derived from the first branchial arch, arises from an ectomesenchymal cell population of cranial neural crest cells (Chai et al., 1998; Hall, 1980). It contains three distinct regions, each having a different fate. The distal region of Meckel's cartilage contributes to mandibular development and undergoes endochondral-like ossification. This region fuses with the rostrum during an early developmental stage and, subsequently, the medial part of the rostrum gives rise to the symphysis region of the mandible. The mid region, the cartilage distal to the ossification center of the mandibular angle, gives rise to the sphenomandibular ligament. Finally, the most proximal region of Meckel’s cartilage, which gives rise to the malleus and incus, also undergoes endochondral ossification (Frommer and Margolies, 1971; Savostin-Asling and Asling, 1973).
The coronoid, condylar and angular cartilage are classified as secondary cartilage (Beresford, 1975). The early mandible bone is formed by intramembranous ossification, but like the epiphyseal cartilage, its proximal end contributes to endochondral components at later stages. The condylar cartilage also serves as an important growth center in the proximal part of the developing mandible (Lee et al., 2001).
Members of the transforming growth factor-β (TGF-β) superfamily mediate a wide range of biological activities, including cell proliferation, differentiation and extracellular matrix formation, suggesting that TGF-β signaling is important during embryogenesis (Chai et al., 2003). TGF-β signaling stimulates proliferation and inhibits terminal differentiation of chondrocytes during chondrogenesis (Alvarez et al., 2001; Serra et al., 1997). We have previously reported the dynamic distribution of cranial neural crest cells (CNC) during Meckel’s cartilage development and the biological function of TGF-β signaling in regulating the proliferation of CNC-derived chondrocytes (Ito et al., 2002). Mice with Tgfbr2 conditional gene ablation in the CNC (Tgfbr2fl/fl;Wnt1-Cre) have craniofacial anomalies including defects in mandible development (Ito et al., 2003). Specifically, these mice have small mandibles, diminished coronoid and condylar processes and missing angular processes. Despite these observations, the downstream mediators of TGF-β signaling to regulate mandible development have remained unclear.
Connective tissue growth factor (CTGF, CCN2) is a member of the CCN family of secreted proteins (Perbal, 2004). CTGF is involved in a variety of developmental processes, including cell determination and differentiation, morphogenesis, and cell migration and proliferation. CTGF is believed to be a downstream mediator of TGF-β action (Chaqour and Goppelt-Struebe, 2006; Grotendorst, 1997), because TGF-β induces the expression of CTGF in fibroblasts (Kikuchi et al., 1995) and chondrocytes (Nakanishi et al., 1997; Shimo et al., 2004) and the CTGF promoter sequence contains a TGF-β response element (Grotendorst et al., 1996; Takigawa et al., 2003). Recently, a study has shown that Ctgf mutant mice exhibit deformation of Meckel’s cartilage and shortened mandibles (Ivkovic et al., 2003). These mandible phenotypes are quite similar to those of Tgfbr2fl/fl;Wnt1-Cre mice.
Here we examine Tgfbr2fl/fl;Wnt1-Cre mice in order to elucidate the role of TGF-β and CTGF in mandible development. We find that the cell proliferation of chondrocytes in Meckel’s cartilage and of the oseteogenic front of the mandibular bone is reduced. Ctgf mRNA expression is decreased in the perichondrium of Meckel’s cartilage. Strikingly, exogenous CTGF rescues the cell proliferation defect in Meckel’s cartilage of the Tgfbr2fl/fl;Wnt1-Cre mutant. Msx1 expression is also reduced in the mandibular primordium of Tgfbr2fl/fl;Wnt1-Cre mice. Finally, the condylar process in Tgfbr2fl/fl;Wnt1-Cre mice has lost the zonation typical of endochondral ossification. This defect begins during the initiation stage of condylar development. Moreover, there are no chondrocytes in the angular process of mandible in the Tgfbr2 mutant. Collectively, our study provides evidence for the functional significance of TGF-β signaling during mandibular development. Tgfbr2fl/fl;Wnt1-Cre conditional knockout mice can serve as a useful animal model that will facilitate a comprehensive understanding of normal craniofacial development as well as CNC-related congenital malformations.
Materials and Methods
Generation of Wnt1-Cre;R26R and Tgfbr2fl/fl;Wnt1-Cre mutant mice and histological analysis
The Wnt1-Cre transgenic line and R26R conditional reporter allele have been described previously (Danielian et al., 1998; Soriano, 1999). We crossed Wnt1-Cre and R26R mice to produce genetically labeled neural crest cells that could be identified with β-galactosidase (Chai et al., 2000). Mating Tgfbr2fl/+;Wnt1-Cre with Tgfbr2fl/fl mice generated Tgfbr2fl/fl;Wnt1-Cre null allele progeny that were genotyped using PCR primers as previously described (Chytil et al., 2002). Samples with no detected Cre transgene were used as controls. All samples were fixed in 4% paraformaldehyde and processed into serial paraffin sections using routine procedures. For general morphology, deparaffinized sections were stained with Hematoxylin and Eosin or Safranin-O staining.
Cryostat sectioning for X-gal staining
Mouse embryonic tissue was frozen, sectioned and then stained according to standard procedures. Specifically, mouse tissue was dissected in PBS and fixed by immersion in 0.2% glutaraldehyde solution overnight at 4°C. Tissue was soaked in 10% sucrose in PBS for 30 minutes at 4°C, incubated in PBS plus 2 mM MgCl2, 30% sucrose, and frozen in OCT. Sections were cut at 8–12 μm and mounted on polylysine-coated slides. The mounted tissue sections were post-fixed in 0.2% glutaraldehyde for 10 minutes on ice, rinsed briefly in PBS and rinsed in detergent solution (0.005% NP-40 and 0.01% sodium deoxycholate in PBS) for 10 minutes at 4°C. Thereafter, the slides were washed in PBS for 10 minutes and stained in X-gal staining solution overnight at 37°C in the dark. Sections were counterstained with Nuclear Fast Red.
Analysis of cell proliferation and apoptosis
DNA synthesis activity within Meckel’s cartilage and mandibular bone was monitored by intraperitoneal BrdU (5-bromo-2′-deoxy-uridine, Sigma) injection (100μg/g body weight) at E11.5, 12.5 and 13.5. One hour after injection, mice were sacrificed and embryos were fixed in 10% buffered formalin solution and processed. Serial sections of the specimen were cut at 7 μm intervals. In each embryonic sample, three randomly selected sections from the middle region of mandible were used for cell proliferation or apoptosis analysis. We detected BrdU labeled cells using a BrdU Labeling and Detection Kit by following manufacturer’s protocol (Zymed). We scored the BrdU-positive and the total number of cells within the Meckel’s cartilage and mandible bone. Student’s t-test was applied for statistical analysis. A p value of less than 0.05 was considered statistically significant. TUNEL assay was performed using the In Situ Cell Death Detection (fluorescein) Kit (Roche Molecular Biochemicals) by following the manufacturer’s protocol.
In situ hybridization
We performed in situ hybridizations following standard procedures. PFA (4%)-fixed samples were dehydrated by passage through a graded ethanol series. The dehydrated samples were subsequently embedded in paraffin in preparation for non-radioactive and radioactive in situ hybridization. Serial tissue sections were treated with proteinase K for 15 min at room temperature. All riboprobes were generated by in vitro transcription using 33P-UTP–labeled or digoxigenin-UTP-labeled and according to the manufacturer’s instructions (Roche Diagnostics Corp.). Several negative controls (e.g. sense probe and no probe) were run in parallel with the experimental reaction.
Organ culture of wild type and Tgfbr2fl/fl;Wnt1-Cre mutant mandible bone primordium explants
Timed-pregnant mice were sacrificed on post-coital day 12.5 and staged according to external developmental characteristics. BSA and TGF-β2 or CTGF beads were implanted in lateral side of Meckel’s cartilage. Mandible explants (six per treatment group) were cultured for 24 hours in serumless, chemically-defined medium according to standard methods (Ito et al., 2002).
Preparation and introduction of TGF-β or CTGF beads
We used affi-gel blue beads (BioRad), for delivery of TGF-β2. The beads were washed in phosphate-buffered PBS and then incubated for 1 hour at room temperature in 10μg/ml TGF-β2 (R&D). Heparin-acrylic beads (Sigma) were incubated in CTGF (20ng/ml, cell sciences) for 1 hour. Control beads were incubated in 0.1% BSA. In both control and Tgfbr2fl/fl;Wnt1-Cre mutant samples, one side of the mandible was treated with BSA beads and the other side treated with TGF-β2 or CTGF beads.
Real-time quantitative RT-PCR
The mRNA levels of Msx1, Msx2 and Ctgf were analyzed by real time quantitative RT-PCR (Bio-Rad iCycler system). Meckel’s cartilage was dissected at E13.5 and total RNA was extracted. The mRNAs were reverse-transcribed into cDNAs by using SuperScript™ First-Strand (Invitrogen life technologies). The real-time PCR was performed using a SYBR super mix kit (Bio-Rad), running for 40 cycles at 95°C for 15 seconds and 60°C for 45 seconds. The melting curve data were collected to check the PCR specificity. Each cDNA sample was analyzed in triplicate. The threshold cycle (CT) was defined as the fractional cycle number. Gene expression values (relative mRNA levels) are expressed as ratios (differences between the Ct values; ΔCt=Ctinterest-CtGapdh) between the genes of interest (Msx1, Msx2 and Ctgf) and an internal reference gene (Gapdh) that provides a normalization factor for the amount of RNA isolated from a specimen. By using Δ(ΔCt) method, {Δ(ΔCt) = ΔCtmutant-ΔCtcontrol}, the fold change {2− Δ(ΔCt)} was calculated for each control and mutant samples. All data are shown as mean±S.D., Student’s t-test was applied for statistical analysis. A p value of less than 0.05 was considered statistically significant.
Results
TGF-β signaling is required for proper development of Meckel’s cartilage and the mandibular bone
As Wnt1 transgene expression is limited to migrating neural crest cells, we generated mice with conditional inactivation of Tgfbr2 gene (Wnt1-Cre;Tgfbr2fl/fl) in all neural crest derived cells (Ito et al., 2003). All Tgfbr2fl/fl;Wnt1-Cre mutant mice had identical mandible defects, consisting of small mandible bones, diminished condylar and coronoid processes and absent angular processes (Fig. 1A–F). Even as the mandible bone was forming on the side of Meckel’s cartilage at E14.5, its size was already smaller in Tgfbr2fl/fl;Wnt1-Cre mutant samples than that of control (Fig. 1G,H). Furthermore, we observed an abnormal shape of Meckel’s cartilage at this stage, with a curvy form and non-uniform thickness areas (Fig. 1F,H). We performed histological analyses of Meckel’s cartilage in the Tgfbr2fl/fl;Wnt1-Cre mutants at multiple time points in order to characterize this defect. As early as E13.5, we detected a deformed shape of Meckel’s cartilage in Tgfbr2fl/fl;Wnt1-Cre mutant mice (Fig. 1K,L). In addition, the visible layers of the perichondrium in Meckel’s cartilage were disrupted (Fig. 1M,N arrow). At earlier stages, the Meckel’s cartilage in the Tgfbr2fl/fl;Wnt1-Cre mutant sample was indistinguishable from the control samples (Fig. 1I,J).
Figure 1. Developmental defects during the formation of Meckel’s cartilage and the mandible bone in Tgfbr2fl/fl;Wnt1-Cre mice.
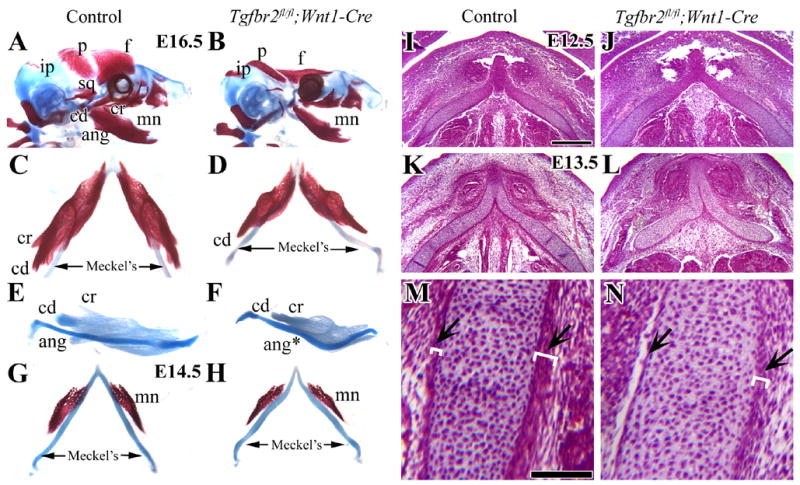
(A,B) Lateral view of skeletal staining preparations of control and Tgfbr2fl/fl;Wnt1-Cre mutant mice at E16.5. Tgfbr2fl/fl;Wnt1-Cre mice have severe defects of the frontal (f), parietal (p) and mandible bones (mn). (C,D) Top view of Meckel’s cartilage and the mandible bone at E16.5 in control and Tgfbr2fl/fl;Wnt1-Cre mutant mice. (E,F) Lateral view of mandible complexes stained with Alcian Blue at E16.5 in control and Tgfbr2fl/fl;Wnt1-Cre mutant mice. Tgfbr2fl/fl;Wnt1-Cre mice have a shortened mandible bone, diminished coronoid and condylar process, and absent angular process. (G,H) Top view of Meckel’s cartilage and the mandible bone at E14.5 in control and Tgfbr2fl/fl;Wnt1-Cre mutant mice samples. (I–M) Histological analysis of Meckel’s cartilage in transversal sections at E12.5 (I,J) and E13.5 (K–N). At E12.5, the Meckel’s cartilage in the Tgfbr2fl/fl;Wnt1-Cre mutant sample is indistinguishable from control. At E13.5, the Tgfbr2fl/fl;Wnt1-Cre mutant sample has defects including a curved Meckel’s cartilage and disrupted layers of the perichondrium and alignment of the chondrocytes in Meckel’s cartilage (arrows). The white parentheses indicate the thickness of the layers. Scale bars: 200μm in I–L; 100μm in M, N.
f; frontal bone, p; parietal bone, ip; interparietal bone, sq; squamous bone, mn; mandible bone, cd; condylar process, cr; coronoid process, ang; angular process, Meckel’s; Meckel’s cartilage
Loss of TGF-β signaling does not affect the migration of CNC cells into Meckel's cartilage and the mandible primordium
In order to test whether a CNC migration defect might contribute to the deficiency of the Meckel’s cartilage and the mandible bone primordium, we generated Tgfbr2fl/fl;R26R;Wnt1-Cre mice. All of these embryos had identical malformations to those seen in the Tgfbr2fl/fl;Wnt1-Cre mutant mice. At E10.5, the first branchial arch had divided into maxillary and mandibular prominences and lacZ expression was detectable in the frontonasal, maxillary and mandibular prominences surrounding the olfactory pit (data not shown). Whole-mount staining has previously shown no difference in migration and distribution of CNC cells within the first branchial arch of Tgfbr2fl/fl;R26R;Wnt1-Cre mutant and control embryos from E9.5 to E11.5 (Ito et al., 2003). After sectioning and staining samples at E12.5, we found that the perichondrium and the chondrocytes of Meckel’s cartilage were primarily composed of CNC-derived cells. The mandibular bone primordium began to form on the lateral side of Meckel’s cartilage (Fig. 2A,B). At E14.5, the mandible primordium had formed bone matrix and the size of the mandible bone in Tgfbr2fl/fl;R26R;Wnt1-Cre mice was smaller than that of R26R;Wnt1-Cre mice. We also observed that the perichondrium of the Meckel’s cartilage was disorganized. However, the contribution of CNC-derived cells in Tgfbr2fl/fl;R26R;Wnt1-Cre was comparable to control (Fig. 2C,D). In addition, we verified the deletion of Tgfbr2 gene in CNC-derived Meckel’s cartilage and mandible. In situ analysis showed that Tgfbr2 was expressed in Meckel’s cartilage and mandible bone primordium in the control sample while it was not detectable in the Tgfbr2fl/fl;Wnt1-Cre mutant (Fig. 2E,F). Taken together, our data suggest that there was no CNC migration defect in the forming Meckel’s cartilage and mandible in Tgfbr2fl/fl;Wnt1-Cre mutant mice.
Figure 2. CNC cell migration into Meckel’s cartilage is not affected by loss of Tgfbr2.
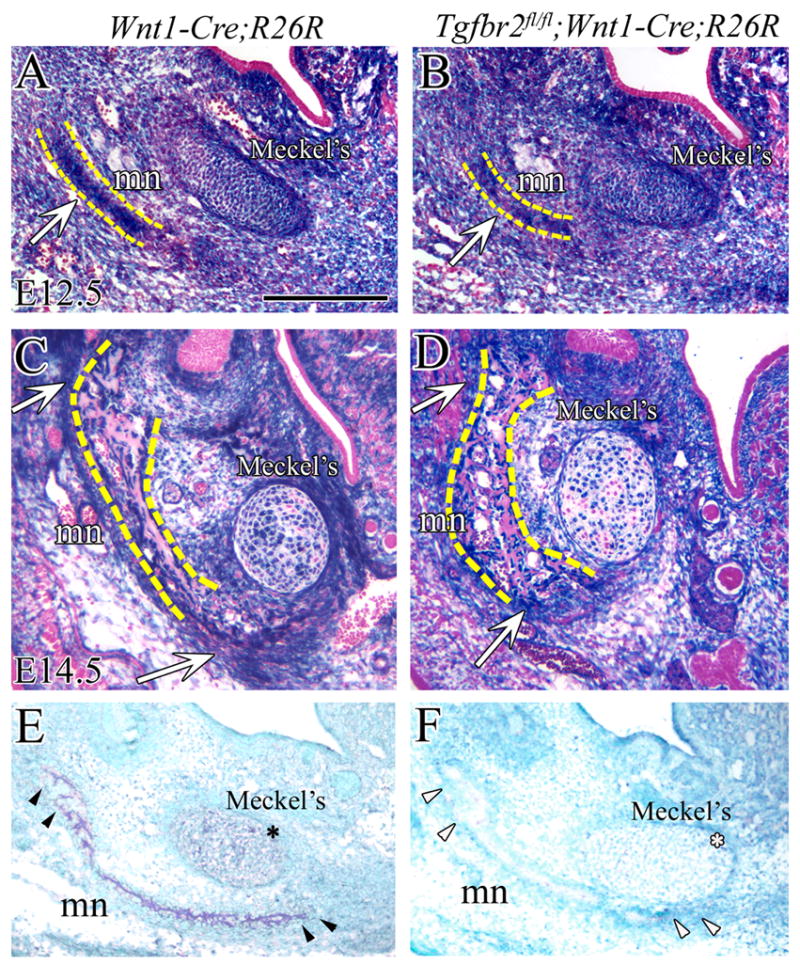
CNC cells are (blue) visualized by X-gal staining of the mandible at E12.5 (A,B) and E14.5 (C,D) in R26R;Wnt1-Cre (control) and Tgfbr2fl/fl;R26R;Wnt1-Cre mice. (A,B) At E12.5, CNC-derived cells are detected in Meckel’s cartilage and the mandible primordium (arrow). There is no apparent difference in the population of cells in Meckel’s cartilage and mandible bone of R26R;Wnt1-Cre and Tgfbr2fl/fl;R26R;Wnt1-Cre mice. (C,D) At E14.5, the Meckel's cartilage is composed of CNC-derived and non-CNC-derived cells. The staining reveals the high density of CNC-derived cells in the osteogenic front of the mandible primordium (arrow). Yellow dashed lines indicate the mandible bone primordium (A,B) or the mandible bone (C,D). (E and F) In situ hybridization shows that Tgfbr2 is expressed (dark blue) in Meckel’s cartilage (*) and in the mandibular primordium (arrowhead) in the control (E) while it is not detectable in the Tgfbr2fl/fl;Wnt1-Cre sample (F) at E13.5. Scale bar: 200μm in A–D. mn; mandible, Meckel’s; Meckel’s cartilage.
Loss of TGF-β signaling perturbs proliferation but not survival of CNC cells
We investigated whether there was a decrease in cell proliferation in Tgfbr2fl/fl;Wnt1-Cre mutant samples by comparing BrdU incorporation in the chondrocytes of Meckel’s cartilage and the osteogenic front of the mandible bone primordium with the control mice (Fig. 3A–F). At E11.5, we did not observe any defects in Tgfbr2fl/fl;Wnt1-Cre mutants by histological analysis. There was no apparent reduction in cell proliferation activity of chondrocytes in Meckel’s cartilage in the Tgfbr2fl/fl;Wnt1-Cre mutant when compared with the control (control, n=6; mutant, n=6; Fig. 3A,B). At E12.5, Meckel’s cartilage in Tgfbr2fl/fl;Wnt1-Cre mutant still did not show any abnormality in form, but we found a significant reduction in cell proliferation activity (p<0.05) within the chondrocytes of Meckel’s cartilage (control, n=6, BrdU index=14.9±0.8%; mutant, n=6, BrdU index=9.1±1.2%; Fig. 3C,D,I). In the mandible primordium, cell proliferation was comparable in the Tgfbr2fl/fl;Wnt1-Cre mutant and control at E12.5. However, at E13.5, cell proliferation activity was significantly reduced in the mandibular primordium of Tgfbr2fl/fl;Wnt1-Cre mutant within both the oral and aboral areas, as compared to control samples (control, n=15; mutant, n=13; Fig. 3E,F). Thus, the reduction in cell proliferation activity occurred before the appearance of the morphological defect in both Meckel’s cartilage and the mandible primordium.
Figure 3.
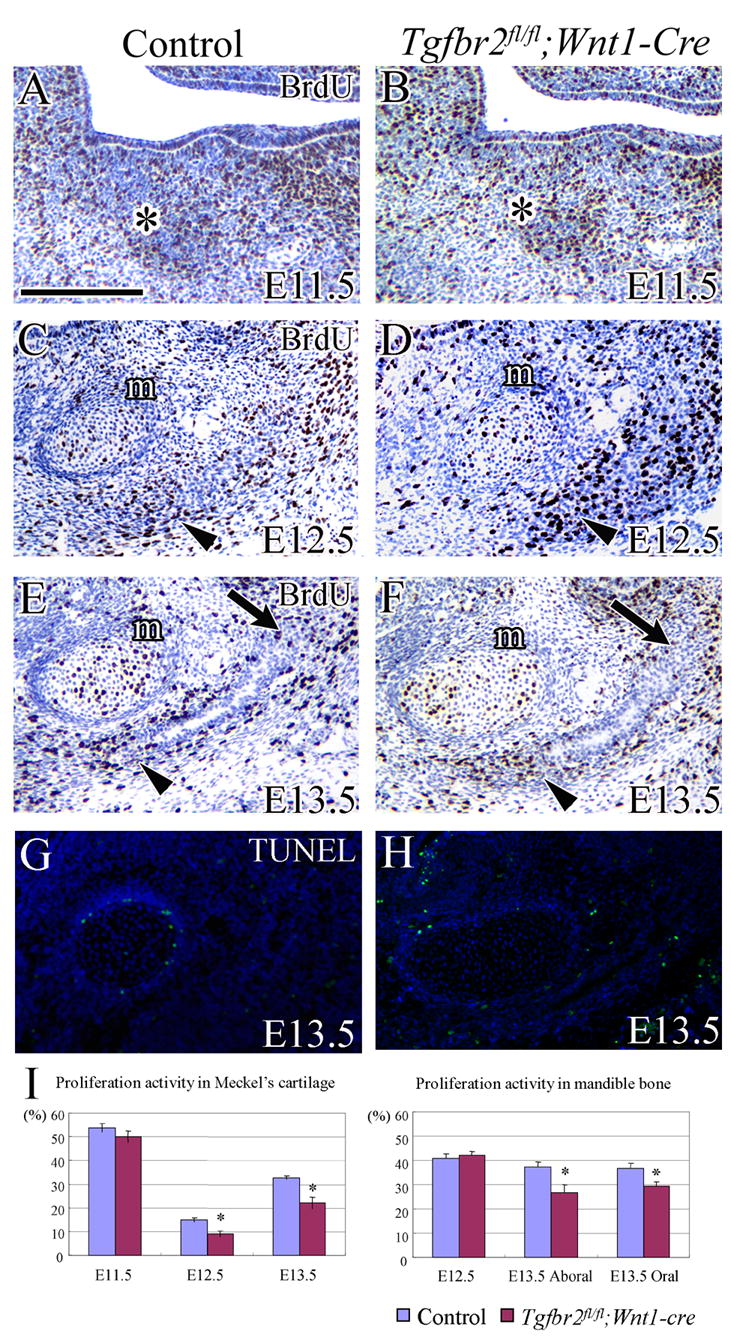
Cell proliferation and apoptosis during Meckel’s cartilage and mandible bone development. (A–F) BrdU incorporation in the chondrocytes of Meckel’s cartilage and the osteogenic front of the mandible bone primordium in control and Tgfbr2fl/fl;Wnt1-Cre mice at E11.5, E12.5 and E13.5. BrdU staining (brown spot) indicates cell proliferation activity. (A,B) At E11.5, Meckel’s cartilage is visible as the highly condensed cell area (asterisk) in both control and Tgfbr2fl/fl;Wnt1-Cre mutant samples. (C,D) At E12.5, the Meckel’s cartilage (m) is well defined and the mandible bone primordium is becoming visible as the highly condensed cell area next to Meckel’s cartilage (arrowhead). (E,F) At E13.5, active CNC cell proliferation is seen in the oral (arrow) and aboral (arrowhead) side of the osteogenic front of the mandible bone, but it is significantly reduced in the Tgfbr2fl/fl;Wnt1-Cre mutant within both the oral and aboral areas. There is also a reduction of proliferation activity in Meckel’s cartilage (m). (G,H) TUNEL assay in the chondrocytes of Meckel’s cartilage and the mandible bone in control and Tgfbr2fl/fl;Wnt1-Cre mice at E13.5. Neither control nor Tgfbr2fl/fl;Wnt1-Cre mice have detectable apoptotic activity. (I) Quantification of cell proliferation activity from A–F. Asterisks indicate statistical significance (p<0.05). Scale bar: 200μm in A–H
To determine whether an increase in apoptosis might have contributed to the defect in mandible development in Tgfbr2fl/fl;Wnt1-Cre mice, we performed TUNEL assays. At E13.5, apoptotic activity was indistinguishable in the Tgfbr2fl/fl;Wnt1-Cre mutant and the control samples (Fig. 3G,H), suggesting that loss of TGF-β signaling did not affect the survival of the CNC-derived mesenchymal cells during mandibular development. Instead, decreased cell proliferation was likely responsible for the defects of Meckel’s cartilage and mandible in the absence of TGF-β signaling.
Downstream mediators of TGF-β signaling in Meckel’s cartilage and mandible bone
To elucidate the molecular signaling cascade involved in regulating cell proliferation of Meckel’s cartilage and mandible bone, we examined the expression of Msx1 and Msx2, genes known to be crucial in regulating mandibular development. At E13.5, Msx1 was expressed around the perichondrium of Meckel’s cartilage and the osteogenic front of the mandible bone and dental mesenchyme in the control (Fig. 4A). In the Tgfbr2fl/fl;Wnt1-Cre mice, Msx1 expression was lost around the perichondrium of Meckel’s cartilage, decreased in the osteogenic front of mandible bone and unchanged in the dental mesenchyme (Fig. 4B). At E14.5, Msx1 expression in control spread to mesenchymal cells between Meckel’s cartilage and mandible bone. However, Msx1 expression was reduced in the Tgfbr2fl/fl;Wnt1-Cre mice (Fig. 4C,D). Msx2 was expressed in the perichondrium of Meckel’s cartilage and the mandible bone primordium in control mice at E13.5 and E14.5. The expression of Msx2 was slightly decreased in the perichondrium of Meckel’s cartilage in the Tgfbr2fl/fl;Wnt1-Cre mice, but was indistinguishable from control in the mandible bone (data not shown). We performed a quantitative analysis of Msx1 and Msx2 expression using dissected Meckel’s cartilage at E13.5 and found that Msx1, but not Msx2, expression was significantly decreased (p<0.01) in the Tgfbr2fl/fl;Wnt1-Cre mice (Fig. 4E and data not shown).
Figure 4. Msx1 expression is down regulated in the Meckel’s cartilage and mandible bone of Tgfbr2fl/fl;Wnt1-Cre mice.
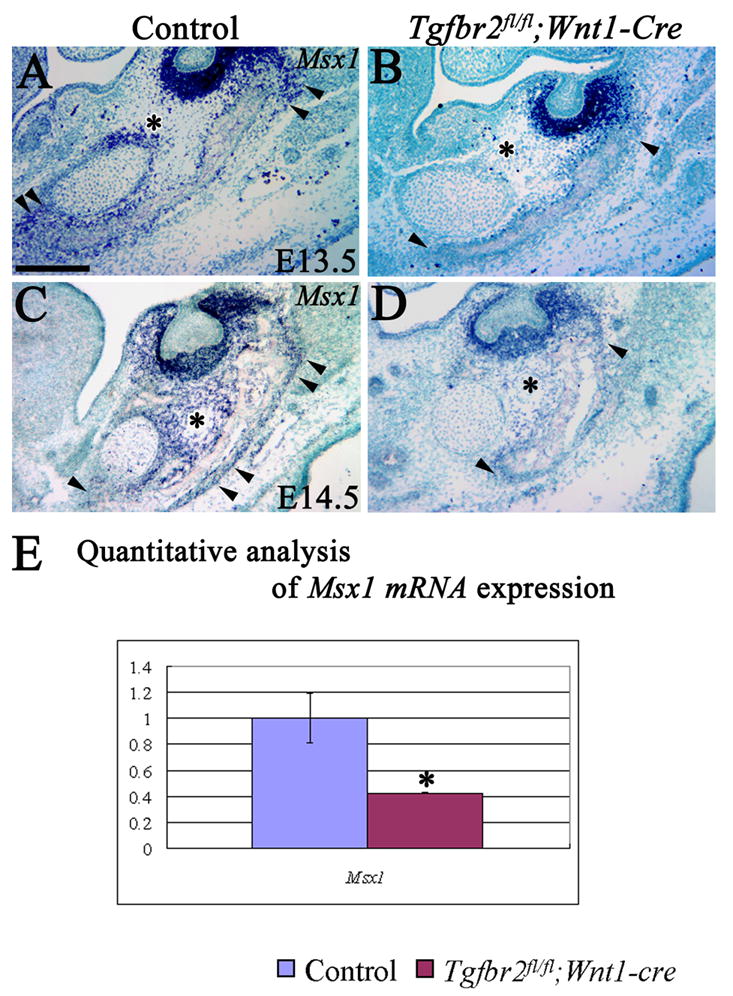
(A–D) In situ hybridization of Msx1 mRNA within Meckel’s cartilage and the osteogenic front of the mandible bone (arrowhead) in control and Tgfbr2fl/fl;Wnt1-Cre mice at E13.5 and E14.5. (*) indicates Msx1 expression in A&C and no Msx1 expression in B&D. (E) Quantitative analysis of Msx1 mRNA expression by real time quantitative RT-PCR in Meckel’s cartilage at E13.5. Asterisks indicate statistical significance (p<0.01). Scale bar: 200μm in A–D
TGF-β signaling and osteoprogenitor differentiation during mandible development
We next analyzed the expression of osteogenic markers to determine if a disruption in osteogenic differentiation might be responsible for the defect in mandible bone development in the Tgfbr2fl/fl;Wnt1-Cre mutant. Runx2 is the earliest identified factor required for osteoblast differentiation, and Twist is a negative regulator of osteoblast differentiation that inhibits Runx2 function (Bialek et al., 2004; Jabs, 2001; Komori et al., 1997; Otto et al., 1997). Runx2 was expressed strongly in the osteogenic front of the mandible bone (Fig. 5A,B). Interestingly, Twist was expressed distinctly on the buccal side of the mandibular bone primordium, in contrast to Runx2 expression (Fig. 5C,D). Runx2 and Twist expression in the developing mandible bone primordium was indistinguishable between control and Tgfbr2fl/fl;Wnt1-Cre mutant samples. Furthermore, we observed no difference in type I collagen (Col I), osterix (Osx), Bsp and osteonectin (ON) expression (Fig. 5E–L). These data suggest that osteoblast differentiation and bone matrix formation appear to be normal in Tgfbr2fl/fl;Wnt1-Cre mutant mice.
Figure 5. TGF-β signaling is not required for bone matrix maturation in the developing mandible bone.
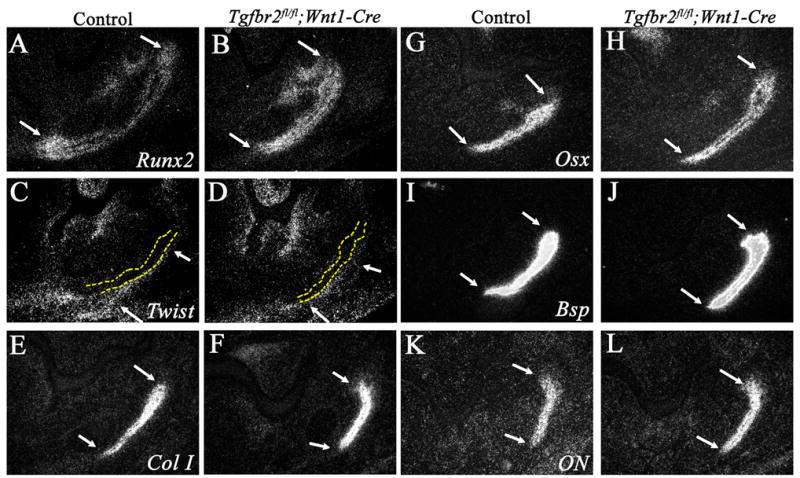
In situ hybridization of the mandible bone primordium at E13.5 in control and Tgfbr2fl/fl;Wnt1-Cre mice with Runx2 (A,B), Twist (C,D) Type I collagen (ColI; E,F), Osterix (Osx; G,H), Bsp (I,J), and Osteonectin (ON; K,L). Arrows indicate the oral (top) and aboral (bottom) sides of the osteogenic front of the mandible bone primordium. Yellow dashed lines (C,D) indicate the mandible bone.
CTGF functions as a downstream mediator of TGF-β signaling to control cell proliferation activity within Meckel’s cartilage
To test whether CTGF is a downstream target of TGF-β signaling during Meckel’s cartilage development, we compared the expression of Ctgf in control and Tgfbr2fl/fl;Wnt1-Cre mutant samples. At E12.5 and E13.5, Ctgf mRNA was specifically expressed in the perichondrium of Meckel’s cartilage in the control, but was reduced in the Tgfbr2fl/fl;Wnt1-Cre mutant (Fig. 6A–D). Next, we performed a quantitative analysis of Ctgf mRNA expression in Meckel’s cartilage at E13.5 using real-time PCR to eliminate the possibility that the reduction of Ctgf expression was due to the disorganization of perichondrium. In fact, Ctgf expression was significantly decreased in Tgfbr2fl/fl;Wnt1-Cre mutant samples (p<0.01) (Fig. 6E), consistent with the proposal that Ctgf is a downstream target of TGF-β signaling in Meckel’s cartilage.
Figure 6. Ctgf expression is down-regulated in the perichondrium of the Meckel’s cartilage in Tgfbr2fl/fl;Wnt1-Cre mice.

(A–D) In situ hybridization of Ctgf in the perichondrium of Meckel’s cartilage at E12.5 and E13.5 in control and Tgfbr2fl/fl;Wnt1-Cre mice. There is a significant reduction of Ctgf expression in Tgfbr2fl/fl;Wnt1-Cre mice at E12.5 and E13.5 (arrowheads, A–D). (E) Quantitative analysis of Ctgf mRNA expression by real time quantitative RT-PCR in Meckel’s cartilage at E13.5. Ctgf expression is dramatically decreased in Tgfbr2fl/fl;Wnt1-Cre mutant samples. Asterisks indicate statistical significance (p<0.01). Scale bar: 200μm in A–D
Differentiation of chondrocytes in Meckel’s cartilage was accelerated in Tgfbr2fl/fl;Wnt1-Cre mice
Ihh is a differentiation marker for maturing chondrocytes, as it is expressed in differentiating chondrocytes and not expressed in proliferating or terminally differentiated chondrocytes. We examined the expression of Ihh in both the control and Tgfbr2 mutant samples. In control samples, Ihh was expressed in the middle region of Meckel’s cartilage (Fig. 7A). However, the area of Ihh expression was expanded in Tgfbr2fl/fl;Wnt1-Cre mice and included the distal part of Meckel’s cartilage (Fig. 7B). This result suggests that the differentiation of chondrocytes in Meckel’s cartilage was accelerated in the Tgfbr2fl/fl;Wnt1-Cre samples. In fact, we did observe abnormal ossification within the Meckel’s cartilage of Tgfbr2fl/fl;Wnt1-Cre mice at birth. In the control sample, Meckel’s cartilage provided the connection between the proximal part of mandible and the middle ear bones (Fig. 7C). In the Tgfbr2fl/fl;Wnt1-Cre mutant sample, there was bone formation around the most proximal part of Meckel’s cartilage that connected to the malleus and incus (Fig. 7D). This data supports the conclusion that differentiation of chondrocyte in Meckel’s cartilage was accelerated in Tgfbr2fl/fl;Wnt1-Cre mutant samples.
Figure 7. Differentiation of chondrocytes in Meckel’s cartilage is accelerated in Tgfbr2fl/fl;Wnt1-Cre.
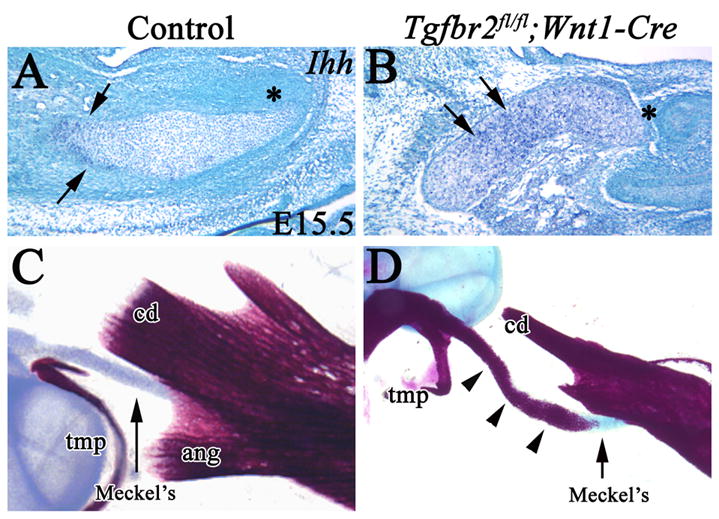
(A,B) In situ hybridization of Ihh in Meckel’s cartilage of control and Tgfbr2fl/fl;Wnt1-Cre mice at E15.5. Ihh is expressed in the middle region (arrow) but not in the distal region (*) of Meckel’s cartilage in control mice. Ihh expression is expanded to the distal region of Meckel’s cartilage in Tgfbr2fl/fl;Wnt1-Cre mice. Asterisks indicate the distal end of Meckel’s cartilage. (C) Skeletal staining of the proximal part of the mandible in the control sample. (D) Skeletal staining of the proximal part of the mandible in Tgfbr2fl/fl;Wnt1-Cre mice. Abnormal ossification (arrowhead) is present around the proximal part of Meckel’s cartilage (arrow). Scale bar: 200μm in A–D. tmp, tympanic ring; cd, condylar process; Meckel’s, Meckel’s cartilage.
Exogenous CTGF protein rescues the cell proliferation defect in Tgfbr2fl/fl;Wnt1-Cre mutants
We hypothesized that TGF-β-mediated CTGF signaling is critical for proliferation of chondrocytes in Meckel’s cartilage. To examine this further, we treated mandible explants from Tgfbr2fl/fl;Wnt1-Cre mutant samples with TGF-β2, CTGF or BSA beads and evaluated CNC cell proliferation activity. As expected, TGF-β2 beads were able to increase cell proliferation activity within Meckel’s cartilage in the control samples, and BSA beads did not (Fig. 8A,C,D). Strikingly, CTGF beads strongly stimulated cell proliferation in Meckel’s cartilage and were able to rescue the cell proliferation defect in the Tgfbr2fl/fl;Wnt1-Cre mutant samples (cell proliferation indices: In the control sample: BSA, 29.25±4.2; In Tgfbr2 mutant sample: BSA, 9.7±2.2; CTGF, 27.3±1.5; p<0.01) (Fig. 8B,E,F), while TGF-β2 failed to rescue the cell proliferation defect in the Tgfbr2 mutant (data not shown). These results strongly suggest that CTGF is a crucial downstream component of the TGF-β signaling cascade regulating CNC cell proliferation during Meckel’s cartilage development (Fig. 8G).
Figure 8. CTGF is a downstream mediator of TGF-β signaling to control cell proliferation in Meckel’s cartilage.
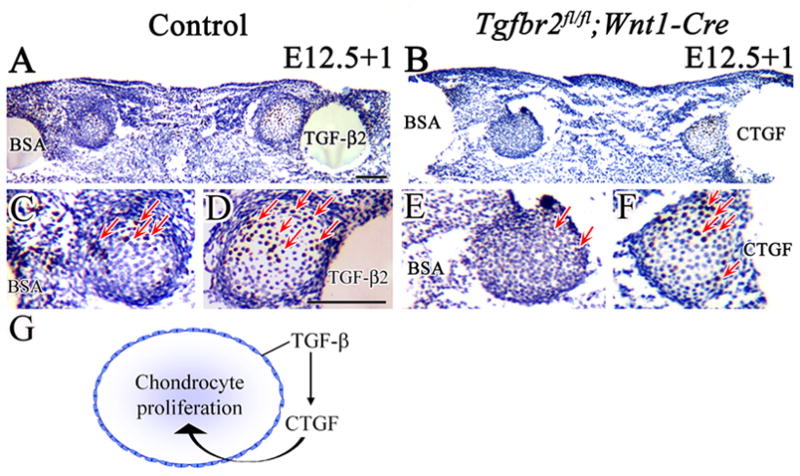
BrdU labeling of cultured Meckel’s cartilage from control and Tgfbr2fl/fl;Wnt1-Cre mice at E12.5, treated with BSA, TGF-β2 or CFGF beads for 24 hours (E12.5+1). (A,C,D) Control sample treated with BSA and TGF-β2 beads. The number of BrdU labeled cells (red arrows) is much greater in Meckel’s cartilage treated with TGF-β2 beads. (B,E,F) Tgfbr2fl/fl;Wnt1-Cre sample treated with BSA and CTGF beads. The Tgfbr2fl/fl;Wnt1-Cre sample has few labeled cells with BSA beads but many more after treatment with CTGF beads (red arrows). (G) Schematic drawing demonstrates that TGF-β controls CTGF expression in the perichondrium to regulate chondrocyte proliferation during Meckel's cartilage development. Scale bar: 100μm in A–F.
TGF-β signaling is required for proper development of the condylar process
In Tgfbr2fl/fl;Wnt1-Cre mutant mice, the condylar and coronoid processes of the mandible bone were defective at E16.5 (Fig. 1A–F). The distribution of CNC-derived cells was widespread in the condylar and angular cartilage, including in the regions of endochondral ossification (Fig. 9A insets). Histological analysis showed that the zonation of endochondral ossification was similar in the condylar and angular processes in the control at E18.5 (Fig. 9A). In contrast, the size of the condylar process in Tgfbr2fl/fl;Wnt1-Cre mutant was smaller than the one in the control samples and the zonation of endochondral ossification was lost (Fig. 9B). At E16.5, we observed well-differentiated chondrocytes and clear zonation, including articular, intermediate, and hypertrophic zones, in condylar processes of control samples (Fig. 9C). In Tgfbr2fl/fl;Wnt1-Cre mutant mice, the chondrogenesis of the condylar processes was diminished and the hypertrophic zone was not present (Fig. 9D, arrow). Prior to cartilage matrix formation, we did observe the initiation stage of the condylar process as mesenchymal cell condensation in the posterior of the ossifying mandible in both control and Tgfbr2fl/fl;Wnt1-Cre mutant samples (data not shown). At E14.5, the condylar cartilage matrix was clearly visible in wild type but not in Tgfbr2fl/fl;Wnt1-Cre mice (Fig. 9E,F). These data suggest that the condylar process defect in Tgfbr2fl/fl;Wnt1-Cre mutant mice was the result of defects in chondrocyte cell fate determination in the proximal part of the mandible.
Figure 9. TGF-β signaling is required for proper development of the condylar process.
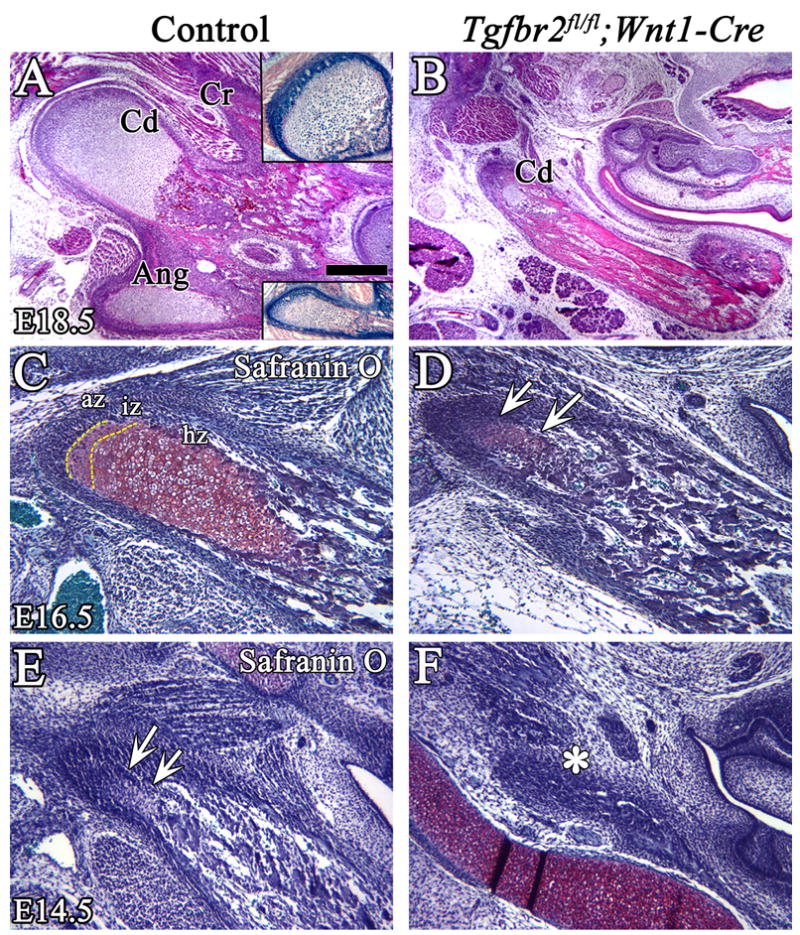
Histological analysis of the coronoid, condylar and angular processes in control and Tgfbr2fl/fl;Wnt1-Cre mice. (A,B) H&E staining shows clear zones of endochondral ossification in the condylar and angular processes in the control at E18.5. The insets show X-gal staining of the condyle and angular processes. The endochondral ossification of the condylar process is diminished in the Tgfbr2fl/fl;Wnt1-Cre mutant at E18.5. (C–F) Safranin O staining of the condyle process at E16.5 (C,D) and at E14.5 (E,F). At E16.5, the three zones, articular, intermediate, and hypertrophic, are visible in the control, but chondrogenesis of the condylar process is dramatically diminished and the hypertrophic zone is not detectable in the mutant (arrows). At E14.5, the condylar cartilage matrix is visible in control (arrows), but not in the Tgfbr2fl/fl;Wnt1-Cre mice (asterisk). Scale bar: 200μm in A–H. Cd; condylar process, Cr; coronoid process, Ang; angular process, az; articular zone, iz; intermediate zone, hz; hypertrophic zone.
Discussion
The mandible bone is formed by intramembranous ossification. Meckel’s cartilage appears to be important in this process as a scaffold and guide for normal mandible development. Meckel’s cartilage development starts as an aggregation of cranial neural crest derived mesenchymal cells at the molar tooth bud region (Ito et al., 2002). Following initiation, Meckel’s cartilage extends towards the anterior and the posterior to develop a “wishbone-like” structure, with CNC-derived cells at the chondrogenic front. Although both CNC- and non-CNC-derived cells contribute to the formation of Meckel’s cartilage, it is clear that CNC-derived cells play an essential role in the development of Meckel’s cartilage and in the guidance of mandible bone formation (Chai et al., 2000; Ito et al., 2002).
In this study, conditional inactivation of Tgfbr2 in neural crest cells results in abnormal formation of Meckel’s cartilage and affects the morphology of mandible development. Of the three mammalian TGF-β isoforms, TGF-β1, -β2, and -β3, only the Tgfb2 knockout mutant has a mandible defect. The mandible does not form a wishbone structure in the Tgfb2 mutant and the coronoid and condylar processes are diminished to approximately one-half their normal dimensions (Sanford et al., 1997). Similarly, conditional inactivation of Alk5 (TGF-β type I receptor) in neural crest cells results in malformation of Meckel’s cartilage and the mandible (Dudas et al., 2006). Furthermore, previous studies have suggested that malformation of Meckel’s cartilage may adversely affect the development of mandible bone. For example, Ctgf−/− mutant mice have an abnormal formation of Meckel’s cartilage and a shortened mandible bone (Ivkovic et al., 2003). Conditional inactivation of Sox9 in the neural crest results in loss of Meckel’s cartilage and malformation of mandible bone (Mori-Akiyama et al., 2003). As Sox9 and Ctgf are not required for intramembranous ossification, the mandible defect in Sox9 and Ctgf mutant mice strongly suggests that Meckel’s cartilage has a critical role in mandible development, working as a guide for the formation of mandible primordium. In addition, loss of Egfr or Shh results in defects in Meckel’s cartilage development and an underdeveloped lower jaw (Melnick et al., 2005; Miettinen et al., 1999). We conclude that growth factor signaling within CNC cells controls the development of Meckel’s cartilage, which has a direct impact on the development of the mandible bone.
TGF-β-mediated Msx1 expression may control cell proliferation in the developing mandible
TGF-β IIR is specifically expressed during the condensation, proliferation and differentiation stages of mandible bone development, suggesting that it has a crucial role in regulating osteogenesis (Janssens et al., 2005). Our Tgfbr2fl/fl;Wnt1-Cre mutant mice have severe craniofacial bone defects in addition to that of the mandible. Previously, we have shown that TGF-β signaling is important for osteoprogenitor cell proliferation in frontal bone development (Sasaki et al., 2006). In the frontal bone primordium, FGF signaling induced by TGF-β is required for osteogenic progenitor cell proliferation. Based on this finding, we examined Fgfr1 and Fgfr2 expression in the mandible of Tgfbr2fl/fl;Wnt1-Cre samples by in situ hybridization, but found no difference from control samples. Previous study has shown that conditional inactivation of Fgfr1 in the neural crest does not affect branchial arch development. Instead, FGFR1 controls the entry of CNC cells into the branchial arch non-cell-autonomously by creating a permissive environment (Trokovic et al., 2003). Taken together, TGF-β signaling within the CNC does not appear to control mandible development via FGFR signaling during craniofacial development.
Members of the Msx homeobox gene family are critical factors involved in craniofacial development. Msx1 null mutant mice have a small mandible and missing alveolar bone (Satokata and Maas, 1994). Loss of Msx1 results in a CNC cell proliferation defect (Han et al., 2003), similar to the cell proliferation defect in the mandible primordium of Tgfbr2 mutant mice. Msx2 null mutant mice do not have a mandible defect (Satokata et al., 2000). Recently, Ishii et al. reported that Msx1/Msx2 double knockout mice have shortened mandibles, which are fused with the maxillary bone (Ishii et al., 2005). Apparently, Msx1 and Msx2 are expressed in pre-migratory and migratory neural crest cells and have major roles in regulating neural crest development (Chai and Maxson, 2006; Gajavelli et al., 2004; Takahashi et al., 2001). Msx genes are known for their essential roles in regulating the fate of CNC cells and osteogenesis during craniofacial development (Han et al., 2003). In this study, we found that Msx1 was expressed in undifferentiated mesenchymal cells between Meckel’s cartilage and the mandible bone at E13.5 and E14.5 in control mice, but was reduced in Tgfbr2fl/fl;Wnt1-Cre mice (Fig. 4). Msx1 may function to induce osteoprogenitor cell proliferation under the control of TGF-β.
TGF-β-mediated CTGF expression controls cell proliferation during Meckel’s cartilage development
TGF-β signaling plays an important role in the regulation of chondrocyte proliferation and differentiation (Serra et al., 1997). Previously, we have shown that TGF-β promotes chondrogenesis by selectively increasing the proliferation of CNC-derived chondrocytes in an in vitro mandibular organ culture model (Ito et al., 2002). In Meckel’s cartilage of Tgfbr2fl/fl;Wnt1-Cre mutant mice, cell proliferation activity was significantly reduced. Other studies have reported that Col2a1-Cre-mediated Tgfbr2 conditional knockout mice do not appear to have any defect of mandible bone development (Baffi et al., 2004; Ovchinnikov et al., 2000). However, the Col2a1-Cre was not expressed in the perichondrium, so TGF-β IIR signaling remained intact there. The perichondrium surrounding the cartilage is thought to synthesize essential factors that regulate cell proliferation and differentiation in chondrogenesis (Lee et al., 2000; Liu et al., 2002; Mukherjee et al., 2005). Based on the differences in craniofacial defects between Tgfbr2fl/fl;Wnt1-Cre and Tgfbr2fl/fl;Col2a-Cre mice, we suggest that TGF-β signaling in the perichondrium plays a critical role in regulating the proliferation of chondrocytes through a possible paracrine mechanism. Thus, loss of TGF-β signaling in the perichondrium results in under-development of the perichondrium and malformation of Meckel’s cartilage.
CTGF is involved in a variety of developmental processes, including cell fate determination, cell migration, proliferation and differentiation (Chaqour and Goppelt-Struebe, 2006; Grotendorst, 1997). CTGF has been proposed as a downstream mediator of TGF-β action because its promoter contains TGF-β response elements (Leask et al., 2003). Loss of Ctgf results in multiple craniofacial defects, including a Meckel’s cartilage malformation that is quite similar to that of the Tgfbr2fl/fl;Wnt1-Cre mutant (Ivkovic et al., 2003). We have detected Ctgf expression in the perichondrium of Meckel’s cartilage at E12.5 and E13.5, and in chondrocytes in Meckel’s cartilage later. Ctgf expression in the perichondrium is significantly reduced in Tgfbr2fl/fl;Wnt1-Cre mutant mice at E12.5 and E13.5. We also observed a reduction in proliferation activity in Tgfbr2fl/fl;Wnt1-Cre mice at E12.5 and E13.5 when Ctgf is expressed specifically in the perichondrium. Finally, we also showed that exogenous CTGF can rescue the cell proliferation defect in Meckel’s cartilage of Tgfbr2fl/fl;Wnt1-Cre mutant samples. These findings suggest that CTGF signaling has a critical role in regulating proliferation of chondrocytes. Thus, we provide the first experimental evidence for a TGF-β-mediated CTGF signaling cascade that regulates craniofacial development.
In addition, we have also investigated the differentiation of chondrocytes in Meckel’s cartilage. We found that Ihh expression, a marker for maturating chondrocytes (Chung et al., 2001; St-Jacques et al., 1999), was expanded in Tgfbr2fl/fl;Wnt1-Cre mice. We conclude that the abnormal formation of Meckel’s cartilage in Tgfbr2fl/fl;Wnt1-Cre mice, is the result of changes in both proliferation and differentiation.
TGF-β signaling and condylar development
During long bone endochondral ossification, TGF-β signaling plays an important role in the regulation of chondrocyte proliferation and hypertrophic differentiation (Serra et al., 1997). The mandibular condylar cartilage, which originates from the mesenchyme of the neural crest, serves as an important growth center in the developing mandible (Noden, 1975; Silbermann et al., 1987). The proximal part of the mandible is composed of the condyle, coronoid, and angular processes, which develop through endochondral ossification, and these three processes are important for mandible function in conjunction with the muscles of mastication. Tgfbr2fl/fl;Wnt1-Cre mice have a defect in all three processes, with a gradient of increasing severity from the distal to the proximal region of the mandible (see Fig 1. E,F). Further study is needed to identify the source and functional mechanism of TGF-β signaling in regulating proximal region of mandible development. Nevertheless, mandible development appears to have a gradient requirement for TGF-β signaling, which functions as a morphogen in regulating mandible formation.
In this study, we focused on the condylar process defect in Tgfbr2fl/fl;Wnt1-Cre mice. This defect started at a very early stage (before E14.5), and the zonation of the condylar process was completely lost at later stages. We found that TGF-β signaling during condylar process development is important for the differentiation of chondrocytes. We also detected changes in Ihh and Pthrp expression in Meckel’s cartilage of Tgfbr2fl/fl;Wnt1-Cre mutant mice. Interestingly, mutants of both of these genes have a defective condylar process (Shibata et al., 2000; St-Jacques et al., 1999 and our unpublished data). Therefore, we hypothesize that TGF-β signaling may control Ihh and Pthrp expression to regulate the development of the condylar process. Further investigation is necessary to explore this issue.
In summary, TGF-β IIR is specifically required for proliferation in both osteoprogenitor and chondroprogenitor cells. TGF-β-mediated CTGF signaling is crucial for the proliferation of CNC-derived chondrocytes in Meckel’s cartilage. Exogenous CTGF can rescue the defect in cell proliferation in Meckel’s cartilage. There are separate signaling pathways that rely on TGF-β signaling to control the proliferation of chondroblasts and osteoblasts during mandible development. Ablation of TGF-β signaling results in a failure of endochondral ossification in the condylar process. This animal model will provide useful information on the mechanism of TGF-β signaling in regulating mandible development.
Acknowledgments
We thank Dr. Julie Mayo for critical reading of the manuscript, Dr. H. Moses for the Tgfbr2fl/fl mice and Drs. Lyons and M. Takigawa for in situ probes and advice on organ culture. This study was supported by grants from the National Institute of Dental and Craniofacial Research, NIH (DE012711, DE014078 and DE017007) to Yang Chai.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez J, Horton J, Sohn P, Serra R. The perichondrium plays an important role in mediating the effects of TGF-beta1 on endochondral bone formation. Dev Dyn. 2001;221:311–21. doi: 10.1002/dvdy.1141. [DOI] [PubMed] [Google Scholar]
- Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R. Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol. 2004;276:124–42. doi: 10.1016/j.ydbio.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Beresford WA. Schemes of zonation in the mandibular condyle. Am J Orthod. 1975;68:189–95. doi: 10.1016/0002-9416(75)90207-9. [DOI] [PubMed] [Google Scholar]
- Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ, Karsenty G. A twist code determines the onset of osteoblast differentiation. Dev Cell. 2004;6:423–35. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- Carda C, Silvestrini G, Gomez de Ferraris ME, Peydro A, Bonucci E. Osteoprotegerin (OPG) and RANKL expression and distribution in developing human craniomandibular joint. Tissue Cell. 2005;37:247–55. doi: 10.1016/j.tice.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Chai Y, Bringas P, Jr, Shuler C, Devaney E, Grosschedl R, Slavkin HC. A mouse mandibular culture model permits the study of neural crest cell migration and tooth development. Int J Dev Biol. 1998;42:87–94. [PubMed] [Google Scholar]
- Chai Y, Ito Y, Han J. TGF-beta signaling and its functional significance in regulating the fate of cranial neural crest cells. Crit Rev Oral Biol Med. 2003;14:78–88. doi: 10.1177/154411130301400202. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–9. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chai Y, Maxson RE., Jr Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353–75. doi: 10.1002/dvdy.20833. [DOI] [PubMed] [Google Scholar]
- Chaqour B, Goppelt-Struebe M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. Febs J. 2006;273:3639–49. doi: 10.1111/j.1742-4658.2006.05360.x. [DOI] [PubMed] [Google Scholar]
- Chung UI, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest. 2001;107:295–304. doi: 10.1172/JCI11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32:73–5. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–6. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Dudas M, Kim J, Li WY, Nagy AJL, Karlsson S, Chai Y, Kaartinen V. Epithelial and ectomesenchymal role of the type I TGF-b receptor ALK5 during facial morphogenesis and palatal fusion. Dev Biol. 2006 doi: 10.1016/j.ydbio.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommer J, Margolies MR. Contribution of Meckel's cartilage to ossification of the mandible in mice. J Dent Res. 1971;50:1260–7. doi: 10.1177/00220345710500052801. [DOI] [PubMed] [Google Scholar]
- Gajavelli S, Wood PM, Pennica D, Whittemore SR, Tsoulfas P. BMP signaling initiates a neural crest differentiation program in embryonic rat CNS stem cells. Exp Neurol. 2004;188:205–23. doi: 10.1016/j.expneurol.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–9. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–80. [PubMed] [Google Scholar]
- Hall BK. Viability and proliferation of epithelia and the initiation of osteogenesis within mandibular ectomesenchyme in the embryonic chick. J Embryol Exp Morphol. 1980;56:71–89. [PubMed] [Google Scholar]
- Han J, Ito Y, Yeo JY, Sucov HM, Maas R, Chai Y. Cranial neural crest-derived mesenchymal proliferation is regulated by Msx1-mediated p19(INK4d) expression during odontogenesis. Dev Biol. 2003;261:183–96. doi: 10.1016/s0012-1606(03)00300-2. [DOI] [PubMed] [Google Scholar]
- Ishii M, Han J, Yen HY, Sucov HM, Chai Y, Maxson RE., Jr Combined deficiencies of Msx1 and Msx2 cause impaired patterning and survival of the cranial neural crest. Development. 2005;132:4937–50. doi: 10.1242/dev.02072. [DOI] [PubMed] [Google Scholar]
- Ito Y, Bringas P, Jr, Mogharei A, Zhao J, Deng C, Chai Y. Receptor-regulated and inhibitory Smads are critical in regulating transforming growth factor beta-mediated Meckel's cartilage development. Dev Dyn. 2002;224:69–78. doi: 10.1002/dvdy.10088. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Jr, Nakajima A, Shuler CF, Moses HL, Chai Y. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development. 2003;130:5269–80. doi: 10.1242/dev.00708. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–91. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs EW. A TWIST in the fate of human osteoblasts identifies signaling molecules involved in skull development. J Clin Invest. 2001;107:1075–7. doi: 10.1172/JCI12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-beta1 to the bone. Endocr Rev. 2005;26:743–74. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Kadono T, Ihn H, Sato S, Igarashi A, Nakagawa H, Tamaki K, Takehara K. Growth regulation in scleroderma fibroblasts: increased response to transforming growth factor-beta 1. J Invest Dermatol. 1995;105:128–32. doi: 10.1111/1523-1747.ep12313452. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Leask A, Holmes A, Black CM, Abraham DJ. Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J Biol Chem. 2003;278:13008–15. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- Lee MC, Goomer RS, Takahashi K, Harwood FL, Amiel M, Amiel D. Transforming growth factor beta one (TGF-beta 1) enhancement of the chondrocytic phenotype in aged perichondrial cells: an in vitro study. Iowa Orthop J. 2000;20:11–6. [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Kim YS, Oh HS, Yang KH, Kim EC, Chi JG. Prenatal development of the human mandible. Anat Rec. 2001;263:314–25. doi: 10.1002/ar.1110. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xu J, Colvin JS, Ornitz DM. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 2002;16:859–69. doi: 10.1101/gad.965602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick M, Witcher D, Bringas P, Jr, Carlsson P, Jaskoll T. Meckel's cartilage differentiation is dependent on hedgehog signaling. Cells Tissues Organs. 2005;179:146–57. doi: 10.1159/000085950. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Chin JR, Shum L, Slavkin HC, Shuler CF, Derynck R, Werb Z. Epidermal growth factor receptor function is necessary for normal craniofacial development and palate closure. Nat Genet. 1999;22:69–73. doi: 10.1038/8773. [DOI] [PubMed] [Google Scholar]
- Mori-Akiyama Y, Akiyama H, Rowitch DH, de Crombrugghe B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc Natl Acad Sci U S A. 2003;100:9360–5. doi: 10.1073/pnas.1631288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Dong SS, Clemens T, Alvarez J, Serra R. Co-ordination of TGF-beta and FGF signaling pathways in bone organ cultures. Mech Dev. 2005;122:557–71. doi: 10.1016/j.mod.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Kimura Y, Tamura T, Ichikawa H, Yamaai Y, Sugimoto T, Takigawa M. Cloning of a mRNA preferentially expressed in chondrocytes by differential display-PCR from a human chondrocytic cell line that is identical with connective tissue growth factor (CTGF) mRNA. Biochem Biophys Res Commun. 1997;234:206–10. doi: 10.1006/bbrc.1997.6528. [DOI] [PubMed] [Google Scholar]
- Noden DM. An analysis of migratory behavior of avian cephalic neural crest cells. Dev Biol. 1975;42:106–30. doi: 10.1016/0012-1606(75)90318-8. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–71. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26:145–6. [PubMed] [Google Scholar]
- Ramaesh T, Bard JB. The growth and morphogenesis of the early mouse mandible: a quantitative analysis. J Anat. 2003;203:213–22. doi: 10.1046/j.1469-7580.2003.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–70. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Ito Y, Bringas P, Jr, Chou S, Urata MM, Slavkin H, Chai Y. TGFbeta-mediated FGF signaling is crucial for regulating cranial neural crest cell proliferation during frontal bone development. Development. 2006;133:371–81. doi: 10.1242/dev.02200. [DOI] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–5. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 1994;6:348–56. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- Savostin-Asling I, Asling CW. Resorption of calcified cartilage as seen in Meckel's cartilage of rats. Anat Rec. 1973;176:345–59. doi: 10.1002/ar.1091760310. [DOI] [PubMed] [Google Scholar]
- Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–52. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Suda N, Yamazaki K, Kuroda T, Beck F, Senior PV, Hammond VE. Mandibular deformities in parathyroid hormone-related protein (PTHrP) deficient mice: possible involvement of masseter muscle. Anat Embryol (Berl) 2000;202:85–93. doi: 10.1007/s004290000100. [DOI] [PubMed] [Google Scholar]
- Shimo T, Kanyama M, Wu C, Sugito H, Billings PC, Abrams WR, Rosenbloom J, Iwamoto M, Pacifici M, Koyama E. Expression and roles of connective tissue growth factor in Meckel's cartilage development. Dev Dyn. 2004;231:136–47. doi: 10.1002/dvdy.20109. [DOI] [PubMed] [Google Scholar]
- Silbermann M, Reddi AH, Hand AR, Leapman RD, Von der Mark K, Franzen A. Further characterisation of the extracellular matrix in the mandibular condyle in neonatal mice. J Anat. 1987;151:169–88. [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–86. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Nuckolls GH, Takahashi I, Nonaka K, Nagata M, Ikura T, Slavkin HC, Shum L. Msx2 is a repressor of chondrogenic differentiation in migratory cranial neural crest cells. Dev Dyn. 2001;222:252–62. doi: 10.1002/dvdy.1185. [DOI] [PubMed] [Google Scholar]
- Takigawa M, Nakanishi T, Kubota S, Nishida T. Role of CTGF/HCS24/ecogenin in skeletal growth control. J Cell Physiol. 2003;194:256–66. doi: 10.1002/jcp.10206. [DOI] [PubMed] [Google Scholar]
- Trokovic N, Trokovic R, Mai P, Partanen J. Fgfr1 regulates patterning of the pharyngeal region. Genes Dev. 2003;17:141–53. doi: 10.1101/gad.250703. [DOI] [PMC free article] [PubMed] [Google Scholar]


