Abstract
In 1991, a potent 61 amino acid vasodilator peptide, named maxadilan, was isolated from the salivary glands of the sand fly. Subsequently, it was shown that this peptide specifically and potently activated the mammalian PAC1 receptor, one of the three receptors for PACAP. These studies and the link between maxadilan and leishmaniasis are discussed.
Keywords: maxadilan, PAC1, receptor, PACAP, leishmaniasis
1. Identification of maxadilan and its mammalian receptor.
Blood-feeding or hematophagous insects express vasoactive compounds in their salivary glands. These compounds serve to counteract the hemostatic processes of the host when the insect obtains a blood meal. The bite of the New World sand fly Lutzomyia longipalpis, a vector of the protozoan disease leishmaniasis, results in the rapid development of a small area of long-lasting redness or erythema at the site (Fig. 1). Erythema is typically associated with vasodilatation. Lerner et al. [4] isolated the vasoactive principle responsible for this effect. Reversed-phase HPLC was used to fractionate sand fly salivary gland extracts. The fractions were injected into the skin of rabbits and activity was determined by the presence or absence of erythema. The erythema-inducing fraction was shown to contain a vasodilatory peptide, named, because of its high potency, maxadilan.
Fig. 1. The sand fly, Lutzomyia longipalpis, taking a blood meal.

Note the area of erythema at the bite site. The local vasodilation is generated by maxadilan, a vasodilatory PAC1-agonist peptide present in the sand fly saliva. This peptide, along with other components of the fly saliva, is injected in the skin during feeding. Image provided courtesy of Jose Ribeiro, National Institute of Health.
The sand fly gene encoding maxadilan was cloned and sequenced a year later [5]. Maxadilan is a vasodilator 500 times more potent than calcitonin gene-related peptide (CGRP) [4], the most potent peptide described at that time. Furthermore, the erythema induced by picogram quantities of maxadilan injected into human skin persists for approximately 48 hours. The vasodilatory properties of maxadilan were subsequently shown to be endothelium-independent and to be, at least in part, mediated by alterations in the levels of intracellular cAMP in smooth muscle cells. In addition to its action on cutaneous vessels, maxadilan also had vasodilatory activity on rabbit aorta and mesenteric artery [2].
Maxadilan was demonstrated to bind to brain extracts from various species including human and mouse [9]. It also bound to neural crest-derived cell lines including the rat pheochromocytoma line PC12, and the human neuroblastoma line NBfl [10]. These results suggested that maxadilan could activate one of the receptors for vasoactive neuropeptides. The CGRP receptors were the initial candidates, because CGRP appeared to induce a similar clinical effect including vasodilation without itching or edema when injected into skin. CGRP and the N-terminus of maxadilan also shared slight sequence similarity. While the receptor for maxadilan was found to be a G-protein coupled receptor (GPCR), is was not that for CGRP, amylin or adrenomedullin. The receptor for maxadilan was found, in 1996, to specifically be the PAC1 receptor. At that time, it was referred to as the PACAP type I receptor. Thus, PACAP27 and PACAP38, but not VIP (a VPAC1 and VPAC2 receptor agonist), inhibited the binding of 125 I- maxadilan to membrane homogenates of rat brain. This discovery was further confirmed in COS cells individually expressing the three types of receptors for PACAP [11] and extended in 2002 with the establishment of a functional assay for PAC1 receptor activation in melanophores [14]. There were two surprising aspect of these ligand — receptor studies. One was that that maxadilan does not share significant primary sequence homology with PACAP (nor VIP), yet these two peptides each activate the PAC1 receptor with essentially equal potency. Second, while PACAP also activates VPAC1 and VPAC2 receptors, maxadilan does not. The activity of maxadilan on various PAC1 splice variants has not been reported.
2. Maxadilan and leishmaniasis
The sand fly is the vector of the protozoan tropical disease leishmaniasis. Leishmaniasis is a major parasitic disease with several hundred thousand cases occurring annually. It is manifest in two forms, a cutaneous form characterized by ulcers on the skin and a visceral form that affects the spleen and, in immunocompromised patients, the bone marrow. The injection of antimony compounds has been the standard treatment for decades although newer compounds have been demonstrated to be effective.
It is known that sand fly salivary gland extracts exacerbate the infectivity of Leishmania major, at least in mice [18, 19]. In addition, Qureshi et al. [15] demonstrated that recombinant maxadilan has immunomodulatory properties. For instance, maxadilan inhibited concanavalin A and anti-TCR antibody — induced T cell proliferation. It also inhibited delayed-type hypersensitivity reactions and mixed lymphocyte reactions. Another study [17] showed that maxadilan decreases the macrophage production of TNF-alpha while stimulating IL-6 production. Maxadilan is thus an immunomodulatory component of the sand fly saliva. Maxadilan has been shown to facilitate the transmission and establishment of leishmaniasis [8] although it is not clear if maxadilan is the only active component of sand fly saliva that exacerbates the infection. When vaccinated against maxadilan, mice are protected against infection with Leishmania major [8]. This fascinating observation leads to the question of whether vaccines that protect against parasites could be directed at components of the parasite vector rather than, or addition to, the parasite.
3. The structure of maxadilan
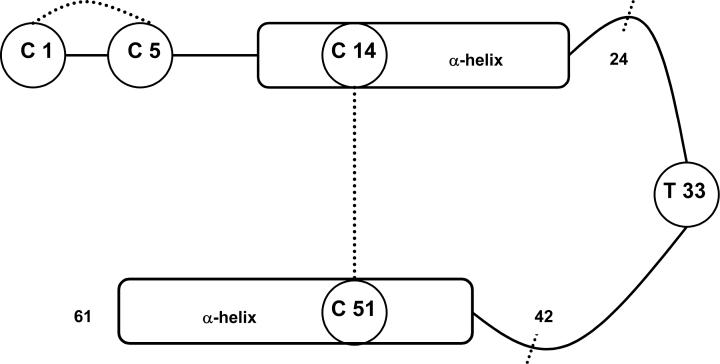
The gene encoding maxadilan contains a single intron present within the signal sequence. The gene predicted a peptide containing 63 amino acids but the predicted mass was at variance, and higher, than the mass determined by mass spectrometry. Following publication of the sequence in 1992, a careful reader contacted us and noted that C-terminal cleavage and amidation often occurred with neuropeptides and that applying this principle to maxadilan would result in a match between the predicted and actual mass. Thus, post-translational processing results in cleavage of both the signal sequence and the C-terminal residues to yield a peptide containing 61 residues (Fig. 2).
Fig. 1. Schematic structure of maxadilan.
The mature 61 amino acid peptide is depicted. Disulfide bonds are present between the cysteines at positions 1−5 and 14−51 and indicated by dashed lines. The predicted alpha helices are labeled. The integrity of the ring between 14−51 is necessary for activity. Deletion of the residues between positions 24 and 42 generated M65, a specific PAC1 receptor antagonist. N-terminal residues appear to be important in receptor binding while a series of threonine residues, particularly that at position 33, is important with respect to receptor activation, as described in the text.
Structure — function aspects of the peptide have been studied by examining a large number of naturally occurring variants as well as synthetic and recombinant deletion and substitution mutants. The activity of natural variants has been determined by erythema induction in rabbit skin. The activity of the initial deletion mutant was determined in binding assays. The activity of all of the many other maxadilans has been evaluated functionally using PAC1 expressing melanophores, an assay that allow for a rapid and reproducible readout [13, 16].
Maxadilan contains four cysteine residues. These form two disulfide rings between the cysteines at positions 1−5 and 14−51. Removal of the first ring or substituting the cysteines in this ring with alanines did not affect activity. Substitution with alanine of the cysteine residues in the second ring, individually or together, resulted in loss of activity. This observation demonstrated the importance of this second ring in maintaining the structural integrity of the peptide in a certain conformation to present necessary residues to the PAC1 receptor.
There is a high degree of sequence divergence in naturally occurring maxadilan depending upon the geographical location of the fly strains. The Lutzomyia longipalpis habitat spreads over a large region in Central and South America, and different sand fly populations are most likely genetically isolated. Lanzaro et al. [3] indeed showed that there was thus up to a 12.8% nucleotide variation between different maxadilan encoding gene sequences and up to 23% amino acid variation in the maxadilan peptide sequences. However, there is no variation in the cysteine residues that constitute the disulfide rings, consistent with the importance of these rings in maintaining structural integrity of the peptide. Maxadilan is predicted through the Chou and Fasman method to contain two alpha- helices connected via a beta-strand motif. Different maxadilans have similar predicted secondary structures and vasodilatory and IL-6 inducing activities. There is however variation in the capacity of saliva from different fly species to induce erythema and enhance leishmaniasis [20]. This may be explained by differences in the quantity of maxadilan present in the saliva of different fly species. A more recent study showed that the maxadilan sequence varies even within the same sand fly population. Different maxadilans have variable antigenic properties and the antigenic specificity appears to be defined by the C-terminal end of the peptide. A successful anti-leishmania vaccine may need to include more than just one immunogenic form of maxadilan [7].
Receptor activation can depend upon both binding and activation domains of the ligand, in this case, maxadilan. Deletion of the amino acids between positions 25 and 41 in the larger disulfide loop generated M65, a potent and specific PAC1 antagonist [12]. We already knew that sequential deletion of residues from the C-terminus resulted in ever-decreasing activity, with complete loss of activity once the cysteine-51 was removed. Since M65 was an antagonist, we reasoned that the C-terminus in general and the several lysine residues in particular could have a role in receptor binding while amino acids in the large loop were important for receptor activation. To further examine this possibility, all of the charged residues in maxadilan were replaced individually and in some cases multiple substitutions were made. While effects on activity were noted, they were not dramatic, leading us to focus on the six threonine residues located in the middle beta-strand that were part of the larger loop. Individual and multiple substitution mutants of the threonine residues were generated. Threonine residues appear to be cooperatively involved in the activation of the PAC1 receptor [16] with threonine-33 being the most important. In cases in which activation no longer occurred, antagonist activity was examined and found to be present in each case, again associated with threonine-33. Of note, substitution of proline-43 did not have a substantial effect on activity.
Taken together, a model was proposed [16] in which C-terminal lysine residues initiate interaction with the PAC1 receptor while threonine residues may be responsible for receptor activation. It is not known if the residues in the PAC1 receptor that are needed for activation are shared by PACAP and maxadilan.
4. Maxadilan as a biological tool
Maxadilan and the antagonist M65 are selective for PAC1 receptors. As such, they should be useful tools for in vitro and in vivo studies in which the contribution of PAC1 receptors can be distinguished from that of other receptors in the same family. Towards that end, maxadilan has been used as a specific activator of PAC1 to investigate the functions of PACAP mediated through the PAC1 receptor in a diversity of physiological settings. For instance, maxadilan, at picomolar concentrations, was shown to alter intracellular calcium levels in pancreatic beta cells. This suggested that PAC1 is, at least in part, responsible for the potentiation by PACAP of glucose-induced insulin release [21]. In the gastric antrum, PACAP-27, PACAP-38 and maxadilan stimulated atrial natriuretic peptide (ANP) secretion from the enterochromaffin cells, while the PAC1 antagonist M65 inhibited basal ANP secretion. This suggested a physiological link between endogenous PACAP and ANP mediated through the PAC1 receptor [1]. Maxadilan has also been used to demonstrate that PAC1 receptors have a role in neural stem cell proliferation [6]. It remains to be seen if PAC1 agonists could be used to relieve the vasoconstriction of Raynaud's Phenomenon or that associated with strokes. In summary, maxadilan and M65 are selective agonists and antagonists respectively of the PAC1 receptor. These peptides could be useful in evaluating the role of the PAC1 receptor as a therapeutic target.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gower WR, Jr, Dietz JR, McCuen RW, Fabri PJ, Lerner EA, Schubert ML. Regulation of atrial natriuretic peptide secretion by cholinergic and PACAP neurons of the gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2003 Jan;284(1):G68–74. doi: 10.1152/ajpgi.00113.2002. [DOI] [PubMed] [Google Scholar]
- 2.Grevelink SA, Osborne J, Loscalzo J, Lerner EA. Vasorelaxant and second messenger effects of maxadilan. J Pharmacol Exp Ther. 1995 Jan;272(1):33–7. [PubMed] [Google Scholar]
- 3.Lanzaro GC, Lopes AH, Ribeiro JM, Shoemaker CB, Warburg A, Soares M, et al. Variation in the salivary peptide, maxadilan, from species in the Lutzomyia longipalpis complex. Insect Mol Biol. 1999 May;8(2):267–75. doi: 10.1046/j.1365-2583.1999.820267.x. [DOI] [PubMed] [Google Scholar]
- 4.Lerner EA, Ribeiro JM, Nelson RJ, Lerner MR. Isolation of maxadilan, a potent vasodilatory peptide from the salivary glands of the sand fly Lutzomyia longipalpis. J Biol Chem. 1991 Jun 15;266(17):11234–6. [PubMed] [Google Scholar]
- 5.Lerner EA, Shoemaker CB. Maxadilan. Cloning and functional expression of the gene encoding this potent vasodilator peptide. J Biol Chem. 1992 Jan 15;267(2):1062–6. [PubMed] [Google Scholar]
- 6.Mercer A, Ronnholm H, Holmberg J, Lundh H, Heidrich J, Zachrisson O, et al. PACAP promotes neural stem cell proliferation in adult mouse brain. J Neurosci Res. 2004 Apr 15;76(2):205–15. doi: 10.1002/jnr.20038. [DOI] [PubMed] [Google Scholar]
- 7.Milleron RS, Mutebi JP, Valle S, Montoya A, Yin H, Soong L, et al. Antigenic diversity in maxadilan, a salivary protein from the sand fly vector of American visceral leishmaniasis. Am J Trop Med Hyg. 2004 Mar;70(3):286–93. [PubMed] [Google Scholar]
- 8.Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol. 2001 Nov 1;167(9):5226–30. doi: 10.4049/jimmunol.167.9.5226. [DOI] [PubMed] [Google Scholar]
- 9.Moro O, Tsomides TJ, Tajima M, Lerner EA. Maxadilan binds to membrane fractions of brain tissue. Biochem Biophys Res Commun. 1995 Nov 2;216(1):234–41. doi: 10.1006/bbrc.1995.2615. [DOI] [PubMed] [Google Scholar]
- 10.Moro O, Tajima M, Lerner EA. Receptors for the vasodilator maxadilan are expressed on selected neural crest and smooth muscle-derived cells. Insect Biochem Mol Biol. 1996 Dec;26(10):1019–25. doi: 10.1016/s0965-1748(96)00025-2. [DOI] [PubMed] [Google Scholar]
- 11.Moro O, Lerner EA. Maxadilan, the vasodilator from sand flies, is a specific pituitary adenylate cyclase activating peptide type I receptor agonist. J Biol Chem. 1997 Jan 10;272(2):966–70. doi: 10.1074/jbc.272.2.966. [DOI] [PubMed] [Google Scholar]
- 12.Moro O, Wakita K, Ohnuma M, Denda S, Lerner EA, Tajima M. Functional characterization of structural alterations in the sequence of the vasodilatory peptide maxadilan yields a pituitary adenylate cyclase-activating peptide type 1 receptor-specific antagonist. J Biol Chem. 1999 Aug 13;274(33):23103–10. doi: 10.1074/jbc.274.33.23103. [DOI] [PubMed] [Google Scholar]
- 13.Nokihara K, Yasuhara T, Nakata Y, Lerner EA, Wray V. Chemical synthesis of maxadilan, a non-mammalian potent vasodilatory peptide consisting of 61 amino acids with two disulfide bridges, and its related peptides. Int J Peptide Res Ther. 2007 June;13(1−2):377–86. [Google Scholar]
- 14.Pereira P, Reddy VB, Kounga K, Bello Y, Lerner E. Maxadilan activates PAC1 receptors expressed in Xenopus laevis melanophores. Pigment Cell Res. 2002 Dec;15(6):461–6. doi: 10.1034/j.1600-0749.2002.02077.x. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi AA, Asahina A, Ohnuma M, Tajima M, Granstein RD, Lerner EA. Immunomodulatory properties of maxadilan, the vasodilator peptide from sand fly salivary gland extracts. Am J Trop Med Hyg. 1996 Jun;54(6):665–71. doi: 10.4269/ajtmh.1996.54.665. [DOI] [PubMed] [Google Scholar]
- 16.Reddy VB, Iuga AO, Kounga K, Lerner EA. Functional analysis of recombinant mutants of maxadilan with a PAC1 receptor-expressing melanophore cell line. J Biol Chem. 2006 Jun 16;281(24):16197–201. doi: 10.1074/jbc.M509429200. [DOI] [PubMed] [Google Scholar]
- 17.Soares MB, Titus RG, Shoemaker CB, David JR, Bozza M. The vasoactive peptide maxadilan from sand fly saliva inhibits TNF-alpha and induces IL-6 by mouse macrophages through interaction with the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor. J Immunol. 1998 Feb 15;160(4):1811–6. [PubMed] [Google Scholar]
- 18.Theodos CM, Ribeiro JM, Titus RG. Analysis of enhancing effect of sand fly saliva on Leishmania infection in mice. Infect Immun. 1991 May;59(5):1592–8. doi: 10.1128/iai.59.5.1592-1598.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988 Mar 11;239(4845):1306–8. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 20.Warburg A, Saraiva E, Lanzaro GC, Titus RG, Neva F. Saliva of Lutzomyia longipalpis sibling species differs in its composition and capacity to enhance leishmaniasis. Philos Trans R Soc Lond B Biol Sci. 1994 Jul 29;345(1312):223–30. doi: 10.1098/rstb.1994.0097. [DOI] [PubMed] [Google Scholar]
- 21.Yamada H, Watanabe M, Yada T. Cytosolic Ca2+ responses to sub-picomolar and nanomolar PACAP in pancreatic beta-cells are mediated by VPAC2 and PAC1 receptors. Regul Pept. 2004 Dec 15;123(1−3):147–53. doi: 10.1016/j.regpep.2004.03.020. [DOI] [PubMed] [Google Scholar]