Abstract
Purpose
Junctional adhesion molecules (JAMs) are a family of adhesion proteins found in intercellular junctions. Evidence suggests that JAM-A is important for the regulation of tight junction assembly and epithelial barrier function. The authors recently reported that JAM-A is expressed in rabbit corneal endothelium and that antibody to JAM-A produces corneal swelling. In the present study, they investigate JAM-A expression in the human corneal endothelium and retinal pigment epithelium (RPE) and examine the effect of a function-blocking antibody to JAM-A on the permeability of cultured RPE cell monolayers.
Methods
Expression of JAM-A in human corneal endothelium, human RPE tissue, and cultured ARPE-19 monolayers was assessed by immunofluorescence confocal microscopy. Localization of JAM-A was compared with the tight junction-associated protein zonula occludens-1 (ZO-1). To investigate JAM-A function in ARPE-19 cells, ARPE-19 monolayers were subjected to a calcium switch protocol to disrupt cell junctions and treated with a function-blocking antibody to JAM-A or an isotype-matched control. Dextran flux assays were performed to assess the effect of JAM-A antibody on ARPE-19 monolayer permeability.
Results
Expression of JAM-A was observed in human corneal endothelium, and its distribution correlated with the tight junction-associated protein ZO-1. In addition, expression of JAM-A was observed in human RPE and in intercellular junctions of ARPE-19 monolayers. The localization pattern of JAM-A in the RPE and ARPE-19 monolayers was similar to that of ZO-1. ARPE-19 monolayers treated with antibody to JAM-A demonstrated a 33% increase in permeability to 10,000 MWt dextran compared with monolayers treated with control antibody.
Conclusions
Results of this study provide new information about JAM-A expression in tight junctions of the human corneal endothelium and human RPE. The observation that antibodies to JAM-A increase ARPE-19 monolayer permeability is consistent with previous findings of JAM-A function in epithelial tight junctions and suggests JAM-A may have a role in the regulation of RPE barrier function.
Junctional adhesion molecules (JAMs) are a family of adhesion molecules expressed in intercellular junctions of epithelial and endothelial cells.1–3 JAMs are also known to be expressed on the surfaces of platelets and leukocytes.3–6 Evidence suggests JAMs are implicated in a variety of cellular adhesive processes. For example, JAM-A is thought to be involved in the regulation of tight junction permeability, leukocyte transmigration, angiogenesis, and platelet aggregation.2–9 Previous studies from our laboratory have shown that antibodies to JAM-A inhibit the recovery of epithelial barrier function after transient calcium depletion.2 Most recently, we reported that JAM-A is expressed in rabbit corneal endothelium and that a function-blocking antibody to JAM-A produces corneal swelling.10
Structurally, JAM proteins are classified within the immunoglobulin superfamily (IgSF) and are characterized by an extracellular domain that contains two immunoglobulinlike loops.1,11 Studies of the crystal structure of JAM-A suggest that JAM-A self-associates to form homodimers and tetramers, and homophilic binding of JAM-A is thought to be important for its function in cells.12–14 In particular, antibodies to JAM-A that block dimerization appear to inhibit the recovery of epithelial barrier function.7 In addition to homophilic binding, it has been suggested that JAMs interact with other classes of adhesion molecules, such as integrins.9,15,16 These heterophilic interactions with integrins are thought to be most relevant to their role in leukocyte transmigration15,16 rather than their role in tight junctions, which is thought to be mediated by homophilic binding.
In addition to extracellular interactions, JAMs contain a short cytoplasmic tail shown to mediate binding scaffold proteins of the postsynaptic density-95/discs large/zonula occludens-1 (PDZ) family. JAM-A has been shown specifically to interact with tight junction-associated PDZ proteins that include zonula occludens-1 (ZO-1), the ALL-1 fusion partner from chromosome 6 (AF-6), calcium/calmodulin-dependent serine protein kinase (CASK), and atypical PKC isotype-specific interacting protein (ASIP).17–19 Although the functional significance of such interactions is not well understood, evidence suggests that they serve as a link to signal transduction pathways critical for the regulation of cell polarity, growth, and differentiation. For example, studies from our laboratory suggest that JAM-A expression regulates epithelial cell morphology, possibly by affecting β1 integrins through the small GTPase Rap1.20 We hypothesize that the link between JAM-A and the Rap1 pathway is mediated by common interactions with PDZ scaffolding proteins.21 Thus, the function of JAM-A in the tight junction appears to involve not only extracellular adhesive interactions but also cytosolic interactions, with PDZ proteins linked to signaling pathways that regulate cell growth and development.
JAM-A has been studied in a variety of epithelial tissues, yet little is known about its expression and function in the human eye. Previously, we reported that JAM-A is expressed in tight junctions of the rabbit corneal endothelium and that a function-blocking antibody to JAM-A produces corneal swelling.10 In this study, we extend these observations by investigating JAM-A expression in the human corneal endothelium and human RPE. We then examine the effects of JAM-A–blocking antibodies on the permeability of RPE cell monolayers. Our results provide new insight into the expression and function of JAM-A in the human corneal endothelium and RPE and suggest that JAM-A may be an important target for the study of epithelial barriers within the human eye.
Materials And Methods
Human Tissue Specimens
Postmortem corneoscleral buttons preserved in storage medium (Optisol-GS; Bausch & Lomb, Rochester, NY) and whole eye globes were obtained from the Georgia Eye Bank (Atlanta, GA) within 3 days of death. All studies were performed with approval of the Institutional Review Board of Emory University.
Antibodies
Mouse monoclonal antibody (clone J10.4) and rabbit polyclonal antibody raised against the extracellular domain of JAM-A were produced as previously described.2,7,10 Mouse monoclonal antibody against human JAM-C (clone PACA-4) was kindly provided by Raven Biotechnologies, Inc. (South San Francisco, CA). Mouse monoclonal antibody against human CAR (clone RmcB) was obtained from Upstate Biotechnology (Lake Placid, NY). Mouse monoclonal antibodies against ZO-1 (clone ZO1–1A12) and occludin (clone OC-3F10) and rabbit polyclonal antibody against claudin-1 were obtained from Invitrogen (Eugene, OR). Alexa Fluor 488– and Alexa Fluor 568–conjugated goat antimouse secondary antibodies were obtained from Invitrogen (Eugene, OR). Alexa Fluor 488–phalloidin was obtained from Invitrogen. Isotype-matched mouse IgG and horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
Cell Culture
The immortalized human RPE cell line ARPE-1922 was obtained from American Type Culture Collection (Manassas, VA). Cells were grown in Dulbecco modified Eagles medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 IU penicillin, 100 μg/mL streptomycin, 15 mM HEPES, and 1% nonessential amino acids (Cellgro, Herndon, VA). Cells were subcultured and harvested with 0.05% trypsin with EDTA in Hanks balanced salt solution. ARPE-19 monolayers were cultured on 6.5-mm transwells with 3.0-μm pore polyester membrane inserts (Corning Life Sciences, Acton, MA), and cultures were maintained at least 6 weeks before use in functional assays.
Immunofluorescence Confocal Microscopy
For analysis of human corneal endothelium, corneoscleral buttons obtained from the Georgia Eye Bank (Atlanta, GA) were fixed in 100% ethanol at −20°C for 20 minutes. Nonspecific antibody binding was blocked by preincubation in 1% bovine serum albumin (BSA) in Hanks balanced salt solution containing calcium and magnesium (HBSS+). Primary antibody against JAM-A was diluted in blocking buffer at a concentration of 2 μg/mL and added to corneas for 1 hour at room temperature. Corneas were washed twice in HBSS+ followed by labeling with secondary antibodies (diluted 1:1000) for 1 hour at room temperature. For double labeling with ZO-1, corneas were incubated for an additional hour in FITC-conjugated ZO-1 antibody (2 μg/mL). Labeled corneas were then washed twice in HBSS+ and mounted in an antifade agent (Prolong Antifade Agent; Invitrogen, Eugene, OR). Immunofluorescence confocal microscopy was performed using a laser scanning microscope (LSM 510; Carl Zeiss Meditec, Inc., Oberkochen, Germany).
For analysis of ARPE-19 cell monolayers, ARPE-19 cells were cultured for six weeks on 6.5-mm transwells with 3.0-μm pore polyester membrane inserts (Product #3472; Corning Life Sciences, Acton, MA). Mature ARPE-19 monolayers were fixed in 100% ethanol at −20°C for 20 minutes, and nonspecific antibody binding was blocked by preincubation in 1% BSA in HBSS+. For the detection of JAM-A, ZO-1, occludin, claudin-1, JAM-C and CAR, primary antibodies were diluted in blocking buffer to final concentrations of 1 to 2 μg/mL. For actin analysis, Alexa 488 –phalloidin was diluted 1:1000 in blocking buffer. Immunofluorescence confocal analysis was performed as described using a laser scanning microscope (LSM 510; Carl Zeiss Meditec, Inc.).
For analysis of human RPE flat mounts, whole globes were obtained from the Georgia Eye Bank (Atlanta, GA) within 3 days of death and stored in moist chambers at 4°C. RPE flat mounts were prepared by microdissection and fixed in 100% ethanol at −20°C for 20 minutes. To quench autofluorescence, RPE flat mounts were pretreated with 0.3% Sudan Black for 7 to 10 minutes and then rinsed with Tris buffer.23 Immunolabeling of JAM-A and ZO-1 was performed as described above, and confocal microscopy was performed using a laser scanning microscope (LSM 510; Carl Zeiss Meditec, Inc.).
Western Blot Analysis
Corneal endothelial cells from human postmortem corneoscleral buttons were separated from Descemet membrane by treatment with trypsin/EDTA. Corneal endothelial cells were then collected by centrifugation and lysed in buffer consisting of 20 mM Tris, 50 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% sodium deoxycholate, 1% Triton X-100, and 0.1% SDS, pH 7.4. Lysis buffer was supplemented with protease and phosphatase inhibitor cocktails containing AEBSF, pepstatin A, E-64, bestatin, leupeptin, aprotinin, microcystin LR, cantharidin, (−)-p-bromotetramisole, sodium vanadate, sodium molybdate, sodium tartrate, and imidazole (1:100 dilution; Sigma, St. Louis, MO). Lysates were then cleared by centrifugation and immediately boiled in reduced SDS sample buffer. SDS-PAGE and immunoblots were performed by standard methods, with antibodies to JAM-A and ZO-1 used at a concentration of 2 μg/mL. For detection of JAM-A and ZO-1 in ARPE-19 cells, cell lysate was prepared from cultures of ARPE-19 cells grown in six-well dishes, and Western blot analysis was performed as described.
Dextran Permeability Assays
Human ARPE-19 cells were cultured for 6 weeks in monolayers on 6.5-mm transwells with 3.0-μm pore polyester membrane inserts (Corning Life Sciences). A “calcium switch” protocol was performed as previously described.2,7 In brief, ARPE-19 monolayers were incubated in calcium-free medium for 1 hour to transiently disrupt cell junctions. Monolayers were then returned to calcium-fortified medium containing either the function-blocking JAM-A antibody J10.4 or an isotype-matched IgG control (20 μg/mL).2,7 After incubation at 37°C for 12 hours, Alexa Fluor-647-labeled dextran (Invitrogen), MWt 10,000, was added to the upper transwell chamber to a final concentration of 0.1 mg/mL, and 50-μl samples were collected from the bottom transwell chamber at various time points thereafter. Fluorescence intensity of each sample was quantified in a microplate reader (FluoStar; BMG LabTech GmbH, Offenburg, Germany). Dextran flux values are reported in arbitrary florescence units (AFUs) and represent an average of six samples obtained at each time point for each group. Error bars represent the SEM for each data point. P values were calculated by Student's t-test.
Results
Localization of JAM-A in the Human Corneal Endothelium
Having previously observed the expression of JAM-A in rabbit corneal endothelium,10 we sought to confirm JAM-A expression in the human corneal endothelium. Flat mounts of human corneal endothelium were prepared from postmortem corneoscleral buttons, and JAM-A expression was assessed by immunofluorescence confocal microscopy. As shown in Figure 1A, expression of JAM-A was observed in intercellular junctions of the human corneal endothelium in a discrete hexagonal pattern similar to that observed in the rabbit corneal endothelium.10 The distribution of JAM-A in the human corneal endothelium was compared with the tight junction-associated protein ZO-1, and significant colocalization of JAM-A and ZO-1 was observed (Fig. 1A, yellow). These findings were also apparent in confocal Z-sections that demonstrated colocalization of JAM-A and ZO-1 in intercellular junctions of the corneal endothelium (Fig. 1A, lower panels). In addition to immunofluorescence detection, Western blot analysis was performed to evaluate JAM-A expression in the human corneal endothelium. As shown in Figure 1B, a single band was detected for JAM-A at the predicted size of 37 kDa. Analysis of ZO-1 expression was included as a positive control, and ZO-1 was band detected at the predicted size of 225 kDa (Fig. 1B). Together these results extend our previous observations of JAM-A expression in the rabbit corneal endothelium and demonstrate expression of JAM-A in the human corneal endothelium in a distribution similar to that of ZO-1.
Figure 1.

Expression of JAM-A in the human corneal endothelium. (A) Immunofluorescence confocal micrographs demonstrate the expression of JAM-A (green) and ZO-1 (red) in human corneal endothelial junctions. Below each en face image is a corresponding Z-section. Note that JAM-A and ZO-1 were observed to colocalize in the junctional complexes (yellow). Scale bar, 10 μm. (B) Western blot analysis of JAM-A expression in human corneal endothelial cells. ZO-1 is shown as a control.
Localization of JAM-A in the Human Retinal Pigment Epithelium
Expression of JAM-A has been detected in a variety of epithelial tissues. Within the eye, epithelial barriers play an important role separating the eye into its distinct physiologic compartments; the corneal endothelium and the RPE are examples of such barriers. Just as the corneal endothelium serves to regulate the diffusion of substances into the corneal stroma and maintain corneal deturgescence, the RPE plays a vital role in the transport of nutrients and metabolites into and out of the neuroretina. In both the corneal endothelium and the RPE, tight junctions form an integral part of the epithelial barrier, and various eye diseases are characterized by the breakdown of tight junctions within these tissues.24–27
Having observed the expression of JAM-A in corneal endothelial cell junctions (Fig. 1), we sought to determine whether JAM-A is expressed in intercellular junctions of the human RPE. Flat mounts were prepared from postmortem human globes, and specimens were fixed and labeled with antibody to JAM-A. Expression of JAM-A in the RPE was assessed by immunofluorescence confocal microscopy. As shown in Figure 2, JAM-A expression was observed in intercellular junctions of the human RPE. Though not identical with ZO-1, JAM-A was observed in a hexagonal pattern and colocalized with ZO-1 in en face images (Fig. 2, top panel) and confocal Z-sections (Fig. 2, bottom panel). More background autofluorescence was noted in JAM-A images, likely from the RPE pigment granules, and this might have accounted for some of the apparent differences in the immunolabeling patterns of JAM-A and ZO-1. Overall, our findings support previous studies of JAM-A epithelial cell junctions and provide new evidence of JAM-A expression in the human RPE.
Figure 2.
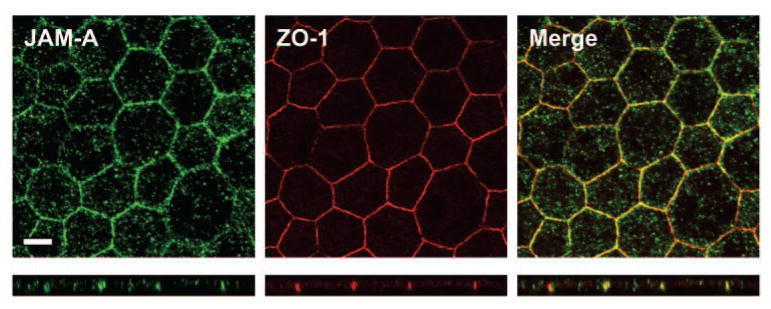
Localization of JAM-A in intercellular junctions of the human RPE. Immunofluorescence confocal microscopy was performed to investigate expression of JAM-A (green) in human RPE flat mounts. The junction-associated protein ZO-1 is shown for comparison (red). Note that JAM-A and ZO-1 were observed to colocalize in junctional complexes (yellow). Below each en face image is a corresponding Z-section. Scale bar, 10 μm.
JAM-A Expression in Cultured ARPE-19 Monolayers
ARPE-19 is an immortalized human RPE cell line commonly used as a model system for studying the RPE.22 Having observed JAM-A expression in human RPE tissue (Fig. 2), we sought to investigate JAM-A expression in cultured ARPE-19 cells. As shown in Figure 3A, immunofluorescence confocal micrographs demonstrate JAM-A labeling in intercellular junctions of ARPE-19 monolayers. JAM-A was observed to colocalize with the intercellular junction-associated protein ZO-1 (Fig. 3A, yellow). Localization of JAM-A in intercellular junctions was apparent in en face images (Fig. 3A, top panel) and confocal Z-sections (Fig. 3A, bottom panel). In addition to immunofluorescence analysis, Western blot analysis was performed to confirm JAM-A expression in ARPE-19 cells. As shown in Figure 3B, a band was detected at the predicted size of 37 kDa for JAM-A. These findings confirm the expression of JAM-A in ARPE-19 cells and support reports that it localizes in intercellular junctions of ARPE-19 cells.28
Figure 3.

Expression of JAM-A in cultured ARPE-19 cells. (A) Immunofluorescence confocal micrographs of cultured ARPE-19 monolayers illustrate JAM-A (green) and ZO-1 (red) expression in intercellular junctions. Colocalization of JAM-A and ZO-1 is shown in yellow. Below each en face image is a corresponding Z-section. Scale bar, 10 μm. (B) Western blot analysis of JAM-A in ARPE-19 cells. ZO-1 is shown as a control.
Heterogeneity of ARPE-19 Cultures
Evidence suggests that ARPE-19 cells are highly sensitive to culture conditions, and significant heterogeneity within ARPE-19 cultures has been observed.28 Therefore, before conducting functional assays, we performed experiments to assess the heterogeneity and maturity of our ARPE-19 cultures. ARPE-19 monolayers cultured for a minimum of 6 weeks were fluorescently labeled with phalloidin, and the actin distribution was assessed by immunofluorescence confocal microscopy. As shown in Figure 4A, some of the actin was observed in circumferential bands that colocalized with JAM-A in apical junctions (shown in yellow). Large stress fibers, however, were also noted within the apical plane and the basal surface. The distribution of actin was more clearly visualized in the enlarged confocal Z-section as shown in Figure 4B, which demonstrates actin filaments in distinct apical, basal, and perijunctional locations. These observations agree with those reported by Luo et al.,28 who also observed significant heterogeneity of actin in ARPE-19 cultures.
Figure 4.

Analysis of actin organization in ARPE-19 monolayers. (A) Immunofluorescence confocal micrographs illustrate the distribution of actin (green) and JAM-A (red) in ARPE-19 cells. Below each en face image is a corresponding confocal Z-section. Scale bar, 10 μm. (B) An enlarged confocal Z-section illustrates the distinct apical and basal actin populations (green) and the perijunctional actin that partially colocalizes with JAM-A (yellow). Note that the vertical scale of this image was increased to allow better discrimination of the apical and basal actin.
In addition to analysis of the actin distribution, experiments were performed to assess the expression of specific tight junction markers in ARPE-19 cultures. Having already observed the expression of the tight junction marker ZO-1 in ARPE-19 cells (Fig. 3), experiments were performed to investigate the expression of two other canonical tight junction proteins, occludin and claudin-1. As shown in the Figure 5A, occludin and claudin-1 were detected by immunofluorescence confocal microscopy in cell junctions of ARPE-19 monolayers. Two close relatives of JAM-A, JAM-C and CAR, were also detected in ARPE-19 cell junctions (Fig. 5B). These results suggest that the ARPE-19 cells express various markers of junctional maturity, but the observed heterogeneity of the actin filaments (Fig. 4) suggests that the polarity may not be uniform throughout the monolayers. Despite these known limitations, the presence of JAM-A and other tight junction proteins in ARPE-19 cells suggest that they may serve as a useful system for investigation of JAM-A function.
Figure 5.
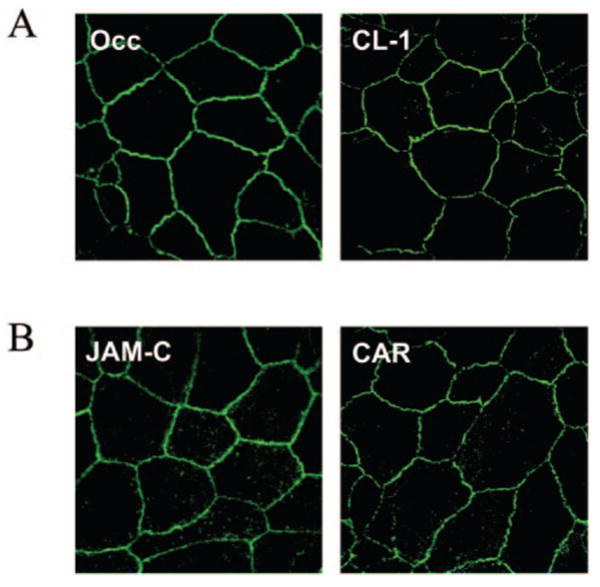
Markers of tight junction maturity in ARPE-19 cells. Immunofluorescence confocal microscopy was performed to assess the expression of the tight junction markers in ARPE-19 cell monolayers. (A) Detection of the canonical tight junction proteins occludin (Occ) and claudin-1 (CL-1). (B) Detection of JAM-A–related proteins JAM-C (JAM-C) and CAR (CAR), two IgSF proteins also commonly expressed in epithelial tight junctions.
Effects of JAM-A Antibody on ARPE-19 Monolayer Permeability
Evidence from multiple studies implicates JAM-A in the regulation of epithelial barrier function. Antibodies to JAM-A have been shown to inhibit the recovery of epithelial barrier function after transient calcium depletion.2,7,8 Therefore, we sought to determine whether antibody to JAM-A affects permeability of ARPE-19 monolayers. ARPE-19 monolayers cultured for 6 weeks were subjected to a “calcium switch” protocol to disassemble cell junctions. They were then treated with antibody to JAM-A or an isotype-matched IgG control, and permeability to 10,000 MWt dextran was quantified fluorometrically at various time points thereafter. As shown in Figure 6A, ARPE-19 monolayers treated with antibody to JAM-A demonstrated significantly greater dextran flux over the time course measured compared with monolayers treated with the control antibody. In fact, an average increase of 33% was observed for monolayers treated with antibody to JAM-A relative to controls. These differences in permeability were statistically significant at P < 0.005 (Fig. 6A). Permeability of ARPE-19 monolayers was also assessed using 40,000- and 70,000-MWt dextrans, and similar increases in permeability were observed for monolayers treated with JAM-A antibody (data not shown). These results suggest that the recovery of barrier function after transient calcium depletion is dependent on JAM-A.
Figure 6.

Effect of JAM-A antibody on permeability of cultured ARPE-19 monolayers. ARPE-19 monolayers cultured on a porous substrate were subjected to a “calcium switch” and then incubated with antibody to JAM-A or control IgG. (A) Permeability to 10,000 MWt dextran was measured fluorometrically at various time points thereafter. Dextran flux is reported in AFUs, with error bars representing the SEM at each time point. *Statistically significant differences with P < 0.005. (B) Confocal micrographs demonstrate binding of the JAM-A antibody in intercellular junctions of ARPE-19 cells. No binding to ARPE-19 cells was observed for the IgG control. Scale bar, 10 μm.
Immediately after completion of the barrier function experiments, immunofluorescence confocal microscopy was performed to detect binding of the JAM-A antibody or IgG control to the ARPE-19 cells. As shown in Figure 6B, significant binding of JAM-A antibody was observed in the intercellular junctions of the ARPE-19 monolayers. No binding was observed for the IgG control (Fig. 6B). These results suggest that the effect of JAM-A antibody on ARPE-19 monolayer permeability (Fig. 6A) is attributed to its binding in cell junctions and not to binding outside junctions or nonspecific IgG-mediated interactions. Over all, our results suggest that JAM-A is important for RPE barrier function, and these findings are consistent with other studies that have demonstrated that antibodies to JAM-A increase epithelial permeability.2,7,8
Discussion
JAMs are a family of intercellular junction-associated adhesion molecules expressed in epithelial and endothelial cells and on leukocytes and other cell types.1,29 Evidence suggests that JAMs are involved in regulating a variety of cellular processes, including tight junction assembly, establishment of epithelial cell polarity, leukocyte transmigration, and angiogenesis.3,9 Antibodies to JAM-A have been shown to prevent the recovery of epithelial barrier function after transient calcium depletion.2,7 Most recently, we reported that JAM-A is expressed in tight junctions of the rabbit corneal endothelium and that antibodies to JAM-A promote corneal swelling ex vivo.10 In the present study, we provide new evidence of JAM-A expression in the human corneal endothelium (Fig. 1) and the RPE (Fig. 2). In addition, we analyze JAM-A expression and function in cultured ARPE-19 monolayers (Figs. 3–6). Together these findings suggest that JAM-A may be an important component of intercellular junctions in the human corneal endothelium and RPE.
It has been reported that tight junctions of the corneal endothelium are “leaky” junctions.30 Studies of the ultrastructure of the corneal endothelium suggest that tight junction strands do not completely encircle the entire circumference of each cell.30 Gaps in specific tight junction proteins, such ZO-1, have been observed at Y-junctions, the points where three endothelial cells intersect.31,32 Gaps in JAM-A staining were also observed at Y-junctions in our previous study of the rabbit corneal endothelium,10 but the functional significance of this finding is unknown. Some degree of leakiness of the corneal endothelium is essential for nutrient diffusion into the stroma, but wholesale disruption of corneal endothelial tight junctions is known to result in corneal swelling and opacity.25 In the present study, we observed gaps at Y-junctions of the human corneal endothelium for both JAM-A and ZO-1 (Fig. 1). In addition to discontinuities at Y-junctions, focal disruptions in staining were observed along the lateral edges of human corneal endothelial cells for JAM-A and ZO-1. In fact, the overall morphology of the human corneal endothelial cells appeared to be more heterogeneous than what was observed in the rabbit.10 Although it is difficult to speculate about the significance of such differences, it is likely that factors such as donor age, duration of specimen storage, and presence of donor eye disease contributed to the observed morphologic differences between the human and rabbit corneal endothelium. Regardless of these subtle differences, it is clear that JAM-A is a component of cell junctions in the human corneal endothelium. Further studies are required to understand the role of JAM-A in eye health and disease.
As does the corneal endothelium, the RPE forms an important physiologic barrier that regulates transport into and out of the neuroretina. Although JAM-A expression was previously reported in cultured chick RPE cells and ARPE-19 cells,28,33 the findings presented in Figure 2 provide the first evidence of JAM-A expression in human RPE tissue. These findings are also unique because they demonstrate the use of Sudan Black23 for the reduction of the background autofluorescence of the RPE pigment granules. As illustrated in Figure 2, significant colocalization of JAM-A and ZO-1 was observed in RPE cell junctions in en face images and corresponding Z-sections. To some extent, the staining for ZO-1 was more continuous along cell borders, and more nonspecific background fluorescence was observed in the JAM-A images. The significance of such differences, however, is not apparent and may reflect biological differences in JAM-A and ZO-1 expression in the RPE and experimentally induced phenomenon related to tissue preparation, fixation, and immunolabeling. Further studies are necessary to fully characterize the subcellular distribution of JAM-A in the human RPE and the functional significance of such observations.
The ARPE-19 cell line is a commonly used culture model for study of the RPE. Significant variability in this cell line has been reported with regard to cell passage number and culture conditions.28 In our study, efforts were made to characterize the maturity and heterogeneity of cultured ARPE-19 monolayers grown for a minimum of 6 weeks. In addition to JAM-A and ZO-1, other biochemical markers of mature tight junctions, namely occludin and claudin-1, were detected by immunofluorescence in ARPE-19 monolayers (Fig. 5A), yet analysis of the actin distribution revealed significant disorganization within ARPE-19 monolayers (Fig. 4). Although some actin was present in circumferential bands typical of mature monolayers, large stress fibers were evident in the apical surface, seen in en face confocal images and Z-sections (Fig. 4). These findings suggest incomplete establishment of cell polarity and junction maturation in ARPE-19 monolayers. Such features may explain the relatively low TER of ARPE-19 cultures compared with more differentiated epithelial cell lines that form mature tight junctions.
Current evidence suggests that JAM-A is important for the regulation of tight junction barrier function in a variety of tissues. Antibodies to JAM-A have been observed to inhibit the assembly of epithelial tight junctions, and evidence suggests that this occurs by blocking homodimerization of JAM-A molecules.2,7,8 In our previous study, we reported that antibody to JAM-A labeled rabbit corneal endothelium and promoted corneal swelling induced by transient calcium depletion.10 Here we demonstrate that antibody JAM-A also increased the permeability of cultured ARPE-19 monolayers to dextran macromolecules (Fig. 6). It should be noted that JAM-A has been shown to affect TER in intestinal epithelial cells, but no effect on TER was observed in ARPE-19 monolayers (data not shown). Given that ARPE-19 cells produced relatively low TER (approximately 60 Ù · cm2) compared with other epithelial cell lines, it is predictable that JAM-A antibodies were not observed to further reduce their TER. Given these findings, we conclude that though ARPE-19 appears to be a suitable model for gross measures of tight junction permeability, such as dextran flux, their heterogeneity and low TER limit their usefulness for study of more subtle changes in tight junction structure and function.
The results of this study and others clearly support a role for JAM-A in the regulation of barrier function. Although the mechanisms that underlie JAM-A function are not entirely understood, JAM-A functions appears to involve the formation of homodimers in the tight junction. As shown in Figure 7A, the extracellular domain of JAM-A contains two immunoglobulinlike loops, and a conserved dimerization motif in the N-terminal immunoglobulinlike loop12-14 is predicted to mediate the formation of JAM-A homodimers12 (Fig. 7B). This model is supported by our findings that specific antibodies block JAM-A homodimer formation and inhibit the recovery of barrier function in vitro.7 In addition, mutations within the putative homodimer motif of JAM-A have been observed to affect the localization of JAM-A at cell–cell contacts.7 Though some debate exists regarding the existence of cis versus trans homodimers,12–14 evidence clearly suggests that homodimerization of JAM-A is important for its function in cells.
Figure 7.

JAM-A and its interactions in the tight junction. (A) JAM-A consists of two extracellular immunoglobulinlike loops, a single transmembrane domain, and a short cytoplasmic tail that terminates in a PDZ-binding motif. The N-terminal immunoglobulinlike loop contains a putative dimerization motif. (B) Monoclonal antibodies to JAM-A have been shown to block dimerization of JAM-A and inhibit JAM-A function in epithelial tight junctions.7 (C) The extracellular domain of JAM-A mediates homophilic interactions, and the cytosolic tail is known to bind to PDZ proteins such as ZO-1, AF-6, and Par-3.17–19,34–38 These PDZ proteins are linked to the actin cytoskeleton and presumably serve as docking sites for small GTPases such as Rho, Rac and Rap-1, which regulate junction assembly and cell polarity.
In addition to adhesive interactions mediated by the extracellular domain, JAMs have been reported to interact with intracellular proteins.17–19,34–38 As shown in Figure 7A, the cytoplasmic tail of JAM-A contains a PDZ-binding motif that has been shown to mediate binding to PDZ scaffold proteins such as ZO-1, AF-6, and Par-3.17–19,34–38 These PDZ proteins presumably serve as adaptor proteins within cell junctions and provide docking sites for regulatory proteins, such as small GTPases, that affect cell adhesion and establishment of cell polarity.21,39 The diagram presented in Figure 7C illustrates putative relationships among JAM-A, PDZ proteins, and signaling molecules within the tight junction. Engagement of JAM-A dimers on the cell surface may regulate the spatial proximity of bound PDZ-bound regulatory proteins, thereby affecting specific signaling pathways within that control tight junction assembly and cell polarity. Nonetheless, much of the current evidence for these PDZ-dependent pathways relies on in vitro binding data, and more in vivo studies are required to demonstrate the relationship between JAM-A and specific PDZ-related pathways.
A number of recent studies have been published regarding the phenotype of JAM-A knockout mice, with particular emphasis on the role of JAM-A in neutrophil migration, dendritic cell trafficking and angiogenesis. Kang et al.40 recently reported that mice lacking JAM-A exhibit morphologic defects in the corneal epithelium, but these differences in cell shape were not associated with any effect on corneal wound healing. Similarly, no corneal swelling or defects in transparency were observed in mice lacking JAM-A. These results were unexpected in light of the abundance of in vitro studies in which antibodies to JAM-A have been shown to affect barrier function. The authors speculate, however, that compensatory pathways may be activated in mice lacking JAM-A, and such compensatory pathways might not have been operational in antibody-based studies in which JAM-A expression was intact.40 Indeed, it is difficult to compare the results of in vitro studies in which JAM-A function was transiently disrupted with in vivo studies in which JAM-A was absent throughout development. Further studies are required to identify compensatory pathways that are activated when JAM-A expression is abrogated.
In summary, we provide new evidence that JAM-A is a component of tight junctions in the human the corneal endothelium and RPE. Furthermore, our findings provide insight into the molecular composition and regulation of these important physiologic barriers in the human eye. Such knowledge may lead to novel strategies for the diagnosis and treatment of eye diseases in which the integrity of these barriers is affected.
Acknowledgments
Supported by National Institutes of Health Grants R01EY000933, P30EY006360, R01DK061379, and R01DK72564.
Footnotes
Disclosure: K.J. Mandell, None; L. Berglin, None; E.A. Severson, None; H.F. Edelhauser, None; C.A. Parkos, None
References
- 1.Mandell KJ, Parkos CA. The JAM family of proteins. Adv Drug Deliv Rev. 2005;57:857–867. doi: 10.1016/j.addr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Nusrat A, Schnell FJ, et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113(pt 13):2363–2374. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Padura I, Lostaglio S, Schneemann M, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kornecki E, Walkowiak B, Naik UP, Ehrlich YH. Activation of human platelets by a stimulatory monoclonal antibody. J Biol Chem. 1990;265:10042–10048. [PubMed] [Google Scholar]
- 5.Naik UP, Ehrlich YH, Kornecki E. Mechanisms of platelet activation by a stimulatory antibody: cross-linking of a novel platelet receptor for monoclonal antibody F11 with the Fc gamma RII receptor. Biochem J. 1995;310(pt 1):155–162. doi: 10.1042/bj3100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malergue F, Galland F, Martin F, Mansuelle P, Aurrand-Lions M, Naquet P. A novel immunoglobulin superfamily junctional molecule expressed by antigen presenting cells, endothelial cells and platelets. Mol Immunol. 1998;35:1111–1119. doi: 10.1016/s0161-5890(98)00102-3. [DOI] [PubMed] [Google Scholar]
- 7.Mandell KJ, McCall IC, Parkos CA. Involvement of the junctional adhesion molecule-1 (JAM1) homodimer interface in regulation of epithelial barrier function. J Biol Chem. 2004;279:16254–16262. doi: 10.1074/jbc.M309483200. [DOI] [PubMed] [Google Scholar]
- 8.Liang TW, DeMarco RA, Mrsny RJ, et al. Characterization of huJAM: evidence for involvement in cell-cell contact and tight junction regulation. Am J Physiol Cell Physiol. 2000;279:C1733–C1743. doi: 10.1152/ajpcell.2000.279.6.C1733. [DOI] [PubMed] [Google Scholar]
- 9.Naik MU, Mousa SA, Parkos CA, Naik UP. Signaling through JAM-1 and αvβ3 is required for the angiogenic action of bFGF: dissociation of the JAM-1 and αvβ3 complex. Blood. 2003;102:2108–2114. doi: 10.1182/blood-2003-04-1114. [DOI] [PubMed] [Google Scholar]
- 10.Mandell KJ, Holley GP, Parkos CA, Edelhauser HF. Antibody blockade of junctional adhesion molecule-A in rabbit corneal endothelial tight junctions produces corneal swelling. Invest Ophthalmol Vis Sci. 2006;47:2408–2416. doi: 10.1167/iovs.05-0745. [DOI] [PubMed] [Google Scholar]
- 11.Williams AF, Barclay AN. The immunoglobulin superfamily—domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- 12.Kostrewa D, Brockhaus M, D'Arcy A, et al. X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. EMBO J. 2001;20:4391–4398. doi: 10.1093/emboj/20.16.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prota AE, Campbell JA, Schelling P, et al. Crystal structure of human junctional adhesion molecule 1: implications for reovirus binding. Proc Natl Acad Sci USA. 2003;100:5366–5371. doi: 10.1073/pnas.0937718100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazzoni G, Martinez-Estrada OM, Mueller F, et al. Homophilic interaction of junctional adhesion molecule. J Biol Chem. 2000;275:30970–30976. doi: 10.1074/jbc.M003946200. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham SA, Rodriguez JM, Arrate MP, Tran TM, Brock TA. JAM2 interacts with α4β1: facilitation by JAM3. J Biol Chem. 2002;277:27589–27592. doi: 10.1074/jbc.C200331200. [DOI] [PubMed] [Google Scholar]
- 16.Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Estrada OM, Villa A, Breviario F, Orsenigo F, Dejana E, Bazzoni G. Association of junctional adhesion molecule with calcium/calmodulin-dependent serine protein kinase (CASK/LIN-2) in human epithelial caco-2 cells. J Biol Chem. 2001;276:9291–9296. doi: 10.1074/jbc.M006991200. [DOI] [PubMed] [Google Scholar]
- 18.Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vest-weber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275:27979–27988. doi: 10.1074/jbc.M002363200. [DOI] [PubMed] [Google Scholar]
- 19.Ebnet K, Suzuki A, Horikoshi Y, et al. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) EMBO J. 2001;20:3738–3748. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandell KJ, Babbin BA, Nusrat A, Parkos CA. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on beta1 integrins and Rap1 activity. J Biol Chem. 2005;280:11665–11674. doi: 10.1074/jbc.M412650200. [DOI] [PubMed] [Google Scholar]
- 21.Boettner B, Govek EE, Cross J, Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc Natl Acad Sci USA. 2000;97:9064–9069. doi: 10.1073/pnas.97.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 23.Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47:719–730. doi: 10.1177/002215549904700601. [DOI] [PubMed] [Google Scholar]
- 24.Edelhauser HF. The resiliency of the corneal endothelium to refractive and intraocular surgery. Cornea. 2000;19:263–273. doi: 10.1097/00003226-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Edelhauser HF. The balance between corneal transparency and edema: the Proctor Lecture. Invest Ophthalmol Vis Sci. 2006;47:1754–1767. doi: 10.1167/iovs.05-1139. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto N, de Kozak Y, Jeanny JC, et al. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia. 2007;50:461–470. doi: 10.1007/s00125-006-0539-2. [DOI] [PubMed] [Google Scholar]
- 27.Campbell M, Humphries M, Kennan A, Kenna P, Humphries P, Brankin B. Aberrant retinal tight junction and adherens junction protein expression in an animal model of autosomal dominant retinitis pigmentosa: the Rho(−/−) mouse. Exp Eye Res. 2006;83:484–492. doi: 10.1016/j.exer.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 28.Luo Y, Zhuo Y, Fukuhara M, Rizzolo LJ. Effects of culture conditions on heterogeneity and the apical junctional complex of the ARPE-19 cell line. Invest Ophthalmol Vis Sci. 2006;47:3644–3655. doi: 10.1167/iovs.06-0166. [DOI] [PubMed] [Google Scholar]
- 29.Bazzoni G. The JAM family of junctional adhesion molecules. Curr Opin Cell Biol. 2003;15:525–530. doi: 10.1016/s0955-0674(03)00104-2. [DOI] [PubMed] [Google Scholar]
- 30.Noske W, Fromm M, Levarlet B, Kreusel KM, Hirsch M. Tight junctions of the human corneal endothelium: morphological and electrophysiological features. Ger J Ophthalmol. 1994;3:253–257. [PubMed] [Google Scholar]
- 31.Barry PA, Petroll WM, Andrews PM, Cavanagh HD, Jester JV. The spatial organization of corneal endothelial cytoskeletal proteins and their relationship to the apical junctional complex. Invest Ophthalmol Vis Sci. 1995;36:1115–1124. [PubMed] [Google Scholar]
- 32.Petroll WM, Hsu JK, Bean J, Cavanagh HD, Jester JV. The spatial organization of apical junctional complex-associated proteins in feline and human corneal endothelium. Curr Eye Res. 1999;18:10–19. doi: 10.1076/ceyr.18.1.10.5392. [DOI] [PubMed] [Google Scholar]
- 33.Luo Y, Fukuhara M, Weitzman M, Rizzolo LJ. Expression of JAM-A, AF-6, PAR-3 and PAR-6 during the assembly and remodeling of RPE tight junctions. Brain Res. 2006;1110:55–63. doi: 10.1016/j.brainres.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 34.Hirabayashi S, Tajima M, Yao I, Nishimura W, Mori H, Hata Y. JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol Cell Biol. 2003;23:4267–4282. doi: 10.1128/MCB.23.12.4267-4282.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebnet K, Aurrand-Lions M, Kuhn A, et al. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: a possible role for JAMs in endothelial cell polarity. J Cell Sci. 2003;116:3879–3891. doi: 10.1242/jcs.00704. [DOI] [PubMed] [Google Scholar]
- 36.Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491–497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung AY, Sheng M. PDZ domains: structural modules for protein complex assembly. J Biol Chem. 2002;277:5699–5702. doi: 10.1074/jbc.R100065200. [DOI] [PubMed] [Google Scholar]
- 38.Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277:455–461. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- 39.Gao L, Joberty G, Macara IG. Assembly of epithelial tight junctions is negatively regulated by Par6. Curr Biol. 2002;12:221–225. doi: 10.1016/s0960-9822(01)00663-7. [DOI] [PubMed] [Google Scholar]
- 40.Kang LI, Wang Y, Suckow AT, et al. Deletion of JAM-A causes morphological defects in the corneal epithelium. Int J Biochem Cell Biol. 2007;39:576–585. doi: 10.1016/j.biocel.2006.10.016. [DOI] [PubMed] [Google Scholar]


