Abstract
The siderophore production of the facultative anaerobe Pseudomonas stutzeri, strain CCUG 36651, grown under both aerobic and anaerobic conditions, was investigated by liquid chromatography and mass spectrometry. The bacterial strain has been isolated at a 626-m depth at the Äspö Hard Rock Laboratory, where experiments concerning the geological disposal of nuclear waste are performed. In bacterial culture extracts, the iron in the siderophore complexes was replaced by gallium to facilitate siderophore identification by mass spectrometry. P. stutzeri was shown to produce ferrioxamine E (nocardamine) as the main siderophore together with ferrioxamine G and two cyclic ferrioxamines having molecular masses 14 and 28 atomic mass units lower than that of ferrioxamine E, suggested to be ferrioxamine D2 and ferrioxamine X1, respectively. In contrast, no siderophores were observed from anaerobically grown P. stutzeri. None of the siderophores produced by aerobically grown P. stutzeri were found in anaerobic natural water samples from the Äspö Hard Rock Laboratory.
In order to facilitate iron(III) acquisition, plants and microorganisms, such as fungi and bacteria, produce and excrete strong iron(III) chelators, i.e., siderophores (18, 22, 23, 33, 34). While fungal siderophores bind to iron(III) by hydroxamate ligands, bacterial siderophores are more structurally diverse, and common ligands are catecholates, hydroxamates, and carboxylates (21). The iron(III) stability constants for bacterial siderophores vary in the range of 1020 to 1052 (6). In addition to iron(III), other metals can be complexed by siderophores. For the trihydroxamate siderophore desferrioxamine B, sometimes called proferrioxamine B (10), some actinides have been shown to have stability constants in the same range as the ferric stability constant (1030.6), e.g., 1026.6 with thorium(IV) and 1030.8 with plutonium(VI) (32), while the stability constant for uranium(VI) was lower, i.e., 1018 (2).
Concerning bacteria, there are several reports on siderophore production by Pseudomonas spp. (1, 3, 4, 19). More than 50 structurally related siderophores, i.e., pyoverdins, produced by the fluorescent Pseudomonas spp., especially Pseudomonas fluorescens and Pseudomonas aeruginosa, have been characterized (3). All pyoverdins emit yellow fluorescent light due to the presence of a 5-amino-2,3-dihydro-8,9-dihydroxy-1-H-pyrimido-quinoline-carboxylic chromophore, to which a peptide chain and a carboxyl chain are attached (1, 3). Nonfluorescent Pseudomonas has also been shown to produce siderophores, such as ferrioxamine E, also called nocardamine (Fig. 1), which was produced by one strain of Pseudomonas stutzeri (19). In addition to ferrioxamines, the P. stutzeri strain KC produced a smaller siderophore, i.e., pyridine-2,6-bis(thiocarboxylic acid) (35). Conversely, a catecholate-type siderophore was shown to be produced by another strain of P. stutzeri, which did not produce any hydroxamate siderophores (4).
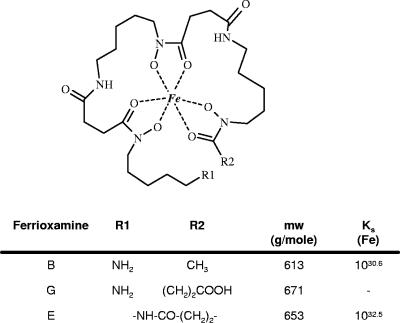
FIG. 1.
Structures, molecular masses (mw), and stability constants (Ks) of ferric complexes of the three ferrioxamines: ferrioxamine B (B), ferrioxamine E (E), and ferrioxamine G (G) (5, 18).
Most of the studies on bacterial siderophore production have been conducted on microorganisms growing under aerobic conditions. One field-based report, however, indicates the occurrence of putative siderophores in anaerobic environments also (29). In the present study, siderophore production has been studied with both aerobic and anaerobic cultures of P. stutzeri. This species is a facultative aerobe, able to grow with oxygen or nitrate as the electron acceptor, meaning that it can be active under both anaerobic and aerobic conditions. The P. stutzeri strain CCUG 36651, studied here, has been isolated from a depth of 626 m below ground at the Äspö Hard Rock Laboratory (16), where research concerning the geological disposal of nuclear waste is performed. The possibility of mobilizing radionuclides by complexing compounds from bacteria is an important research area in the context of nuclear waste disposal research. It is unknown if such compounds are produced in aquifers under conditions relevant to a disposal site, which would be approximately 500 m underground in granitic rock (27).
A study from 2004 shows that P. stutzeri growing aerobically in the presence of uranium-containing shale leached Fe, Mo, V, and Cr from the shale material (17). More recently it was shown that the supernatant of aerobically and anaerobically cultured P. stutzeri was able to increase the partitioning of added Fe, Pm, Am, and Th into the aqueous phase in samples where quartz sand was used as a solid surface (16). Aerobic supernatants maintained 60% or more of the added metals in solution, while anaerobic supernatants were best at maintaining Am in solution, reaching a value of 40% in solution. The increased partitioning to the aqueous phase in the presence of the supernatants was ascribed to the production of organic ligands. Supernatants of both aerobically and anaerobically grown P. stutzeri strain CCUG 36651 yielded a positive response on the universal siderophore assay, the CAS assay (16). This assay is based on ligand competition for iron bound to the colored chrome azurol complex (25, 30).
In this study, siderophore production by P. stutzeri strain CCUG 36651 was investigated using mass spectrometry (MS) and liquid chromatography (LC) followed by mass spectrometric detection. Electrospray ionization mass spectrometry (ESI-MS) and electrospray ionization tandem mass spectrometry (ESI-MS/MS) are useful tools in characterizing siderophores such as ferrioxamines (10, 13, 14, 28, 31). In order to detect iron(III)-chelating compounds, the ferric iron can be replaced by gallium(III) through ascorbate-mediated reduction of iron(III) (8, 20). In mass spectra, gallium-bound substances are easily recognized due to the characteristic isotope pattern of gallium, where the intensity of the 71Ga signal is about 66% of that of the 69Ga signal. The use of ESI provides so-called soft ionization; thus, information about the molecular weight is obtained. However, by employing MS/MS, fragmentation is achieved, providing more information about the compound structure.
In order to verify the chemical difference between the siderophores found by ESI-MS, chromatographic separation was performed. In this case, one reversed-phase C18 column and one column containing a porous graphitic carbon (PGC) stationary phase were used. The separation mechanism of PGC is a combination of hydrophobic interactions, as in C18, and electrostatic interactions between π-electrons. In order to detect substances at low concentrations, column-switched capillary chromatography with MS detection was used. The detection limits of the combined LC-MS/MS system used in this study are in the range of 1 to 5 nM for hydroxamate siderophores of the ferrichrome and ferrioxamine families (9). In order to facilitate analysis of lower concentrations of ferrioxamines, natural water samples were preconcentrated by solid-phase extraction (SPE), resulting in minimum detectable concentrations in the range of 0.02 to 0.1 nM, depending on the initial sample volume.
MATERIALS AND METHODS
Chemicals.
All water used was 18.2-MΩ cm−1 water purified by a MilliQ-purification system (Millipore). For cultivation, analytical-grade K2HPO4, NH4Cl, MgCl2 · 6H2O, CaCl2 · 2H2O, MgSO4 · 7H2O, KNO3, NaOH, Na-lactate (50%), and cysteine-HCl, obtained from Merck, were used. For siderophore extraction and characterization, the following materials were used. Formate buffer (pH 4.0; 11 mM) was prepared in water using formic acid of analytical grade and ammonium formate of analytical grade (Sigma-Aldrich). Methanol and acetonitrile were of high-performance-LC grade (Chromasolv; Sigma-Aldrich). Reference siderophore solutions were prepared using iron-free desferrioxamines, i.e., desferrioxamines E and G (EMC Microcollections, Tübingen, Germany) and desferrioxamine B (Sigma). Iron(III) complexes were prepared by addition of FeCl3 (analytical grade; Merck), while for exchange to gallium(III), Ga(NO3)3 · H20 (>99.9%) and ascorbate, i.e., sodium l-ascorbic acid (99%) (both from Acros Organics), were used.
Media, cultivation conditions, and collection of bacterial supernatants.
Pseudomonas stutzeri (CCUG 36651 [www.ccug.se]), which was isolated from borehole KAS03 at a 626-m depth in the Äspö Hard Rock Laboratory, was grown in batch cultures under aerobic and anaerobic conditions. The basal salt solution of both the aerobic and the anaerobic medium had the following composition: 0.06 mM K2HPO4, 5.6 mM NH4Cl, 0.5 mM MgCl2, 0.7 mM CaCl2, 0.4 mM MgSO4, and 11 mM KNO3. Lactate was added to the autoclaved and cooled medium to be used for aerobic cultures to a final concentration of 4.4 mM, and the pH was adjusted to 8 using 1 M NaOH. The aerobic medium was dispensed in 500-ml Erlenmeyer flasks with 200 ml of medium in each flask. The anaerobic medium was cooled under an N2 atmosphere after autoclaving, and lactate was added to a final concentration of 4.4 mM. Furthermore, the reductant cysteine-HCl was added to a final concentration of 1.5 mM and the pH was adjusted to 8 using 1 M NaOH. The anaerobic medium was dispensed by applying an overpressure to the medium vessel and allowing the medium to flow via an outlet tube and a syringe into serum bottles with an N2 atmosphere. A 100-ml aliquot of medium was dispensed into each bottle. Aerobic flasks and anaerobic serum bottles were inoculated with approximately 100 μl of P. stutzeri growing actively in an aerobic or anaerobic medium of the same type as outlined above. Cultures were grown for 1 week at room temperature on an orbital shaker (Labotron), after which the contents of the multiple flasks were pooled in one aerobic and one anaerobic culture solution. Each culture solution was then centrifuged at 8,000 × g for 10 min in a Sorvall RC-5B Superspeed centrifuge (Thermoelectron Corporation). Finally, each supernatant was suction filtered (Filtropur BT50, 0.2 μm; Sarstedt, Landskrona, Sweden) into a sterile bottle, and the resulting solution was subdivided into 50-ml polypropylene (PP) tubes (Sarstedt) and frozen pending analysis.
Natural water samples from 450 m underground at the Äspö Hard Rock Laboratory.
Four different samples of groundwater were collected at the Äspö Hard Rock Laboratory, which is located on the island of Äspö, southeast Sweden (26). Groundwater was circulated at a flow rate of 30 ml/min via a borehole (KJ0052F01) at the microbe site, located 450 m below ground, via polyether ketone tubing into three circulations (24). The circulation systems were made of polyether ketone and polyvinylidine difluoride plastic; metal was not in contact with the circulating groundwater. Each circulation had four flow cells for attachment and growth of microorganisms (biofilms). The total surface area was 2,112 cm2 per circulation. The groundwater was anaerobic and reduced with populations of sulfate-reducing bacteria, with methanogens and acetogens among the dominating species. The groundwater was circulated through all three circulations set in a series under ambient pressure and temperature, i.e., 30 bars and 17°C. After 1 month, it was collected without coming in contact with air into butyl-rubber stopper-sealed 120-ml sterile serum bottles with an N2 atmosphere. Ferric iron (1.4 μmol) was added to 625 ml of water from the circulation (KJ0052F01) before SPE.
After 6 months of circulation via the borehole, the three circulations were isolated from each other and from the borehole and became closed circulating systems. There was 5,000 ml groundwater circulating at 30 ml/min under in situ conditions in each circulation. Biofilms had developed in the flow cells during the 6 months before closure. The total number of attached and unattached cells in each circulation at closure was approximately 5 × 109 cells. To the first circulation, acetate was added to a concentration of 140 mg/liter; to the second, H2 and CO2 were added to final concentrations of 20 ml/liter and 4 ml/liter, respectively; and the third circulation was used as a control, without any addition. After 3 months of continued circulation under closed conditions, water from each circulation was collected in 50-ml polypropylene tubes from Sarstedt. The tubes were frozen immediately after sampling. To 50 ml of each sample, 0.2 μmol of ferric iron was added before extraction by SPE. At this stage all three circulations had increased their numbers of attached and unattached cells about three times; thus, there was a significant growth in the water and also on the surfaces.
Purification of siderophores in cultures by SPE.
Each SPE cartridge (Sep-Pak C18+, 360 mg; Waters Co.) was activated and cleaned prior to use, first by 10 ml of methanol and then by 2 × 10 ml of milliQ water. Metal-bound siderophores were obtained by addition of either 1 μmol iron(III) or 1 μmol gallium(III), and the sample was drained through the SPE column. Salts were rinsed out by 5 ml of milliQ water, after which the siderophores were eluted by 13 ml of methanol that was subsequently evaporated by means of rotary evaporation under vacuum. The residue was collected, and aqueous ammonium formate buffer (pH 4, 11 mM) was added to a final sample volume of 1.0 ml. The sample was filtered (Millex-GV, 0.22 μm; Millipore) before analysis. Prior to MS and MS/MS experiments, methanol was added to a final concentration of approximately 20% (vol/vol). The extracted sample volume of the anaerobic culture was increased to 100 ml using two cartridges instead of 25 ml using one cartridge as with the aerobic culture.
Exchange of iron to gallium bound to siderophores.
Since iron was not removed from the bacterial culture medium, an ascorbate-mediated exchange of residual iron to gallium was performed in order to ensure that the siderophores were complexed by gallium. To the extracted sample, 1 μmol of ascorbate, 1 μmol of gallium(III), and 20 μmol of formic acid were added, and the mixture was ultrasonicated for 30 min. The sample was then purified by SPE using one cartridge and the same procedure described above for extraction of siderophores from culture samples.
Characterization of siderophores by ESI-MS and ESI-MS/MS.
The molecular masses of the extracted siderophores bound to iron(III) or gallium(III) were determined by ESI-MS, while structural information was obtained by ESI-MS/MS. The instrument used was a triple-quadrupole mass spectrometer (Sciex API 3000; Applied Biosystems) operated in positive ESI mode. Direct infusion at a flow rate of 5 μl/min was performed using a syringe pump (Harvard Apparatus). The mass spectrometer was tuned by direct infusion of 0.5 μM standards of ferrioxamine B, E, and G, resulting in the following MS settings: ion spray potential, 5,000 V; declustering potential, 75 V; focusing potential, 250 V; entrance potential, 10 V. These settings were also used for the MS/MS experiments, where the cell exit potential was optimized to 10 V and the collision gas flow to 7 (arbitrary units of N2), and the collision energy was varied in the range of 30 to 70 V. Reference ESI-MS and ESI-MS/MS spectra were collected using the same ferrioxamine standard solutions. ESI-MS spectra were recorded in the ranges of 200 to 900 atomic mass units (amu) and 200 to 1,600 amu, while ESI-MS/MS spectra were collected between 40 and 700 amu.
LC-ESI-MS and LC-ESI-MS/MS analysis of siderophores.
Separation of siderophores in extracted supernatants was performed by capillary LC followed by ESI-MS or ESI-MS/MS detection. The LC system was composed of one microflow pump (Perkin Elmer 200 Micro Series), an autoinjector equipped with a thermostat (Agilent 1100), and a triple-quadrupole mass spectrometer (Sciex API 3000). Two different columns were used, i.e., one HyPurity C18 column and one Hypercarb PGC column, both having the dimensions 100 by 0.50 mm, 5 μm, and manufactured by Thermo Hypersil-Keystone. Elution of both columns was performed for 40 min at a flow rate of 10 μl/min with a mobile phase containing 5% (vol/vol) acetonitrile in 11 mM ammonium formate buffer, pH 4.0. The injection volume was 2 μl, and the samples were kept at 4°C during analysis. The mass spectrometer was operated in positive ESI scan mode between m/z 550 and 750 amu.
In order to analyze natural water samples on-line, preconcentration by means of column switching was employed. The previously described chromatographic system was used with the further addition of one Perkin Elmer 200 series pump for gradient elution of the analytical column (HyPurity C18, 100 by 0.50 mm, 5 μm) and one microflow pump (Shimadzu LC-10ADvp) for elution of the precolumn (HyPurity Aquastar C18, 30 by 0.50 mm, 5 μm; Thermo Hypersil-Keystone). All mobile phases were prepared by mixing acetonitrile with 11 mM ammonium formate buffer, pH 4.0. The precolumn was eluted with 0.5% (vol/vol) acetonitrile at a flow rate of 10 μl/min. Gradient elution of the combined precolumn and analytical column was achieved using two mobile phases containing 5% (vol/vol) and 50% (vol/vol) acetonitrile at a total flow rate of 10 μl/min. A sample volume of 50 μl was injected onto the precolumn where the siderophores were adsorbed and concentrated, while salts were washed out. After 10 min, column switching was performed by means of a 10-port switch valve (VICI EPC10W; Valco Instruments), and the adsorbed siderophores were transferred from the precolumn to the analytical column by a mobile phase containing 5% (vol/vol) acetonitrile at a flow rate of 10 μl/min. After a total elapsed time of 35 min, the acetonitrile content of the mobile phase flowing through both columns was linearly increased from 5% (vol/vol) to 40% (vol/vol) during the following 20 min. The mobile-phase composition was maintained at 40% (vol/vol) acetonitrile for another 10 min until a total elapsed run time of 65 min, when the precolumn was decoupled from the analytical columns and both columns were regenerated by their initial mobile phases for 60 min prior to the next injection. Detection of two fragments of the four siderophores produced by the aerobically grown P. stutzeri was performed by multiple reaction monitoring of the proton adducts of the ferric complexes on a triple-quadrupole mass spectrometer (Sciex API 3000) operated in positive ESI mode. In addition, detection was also performed in positive scan mode between m/z 550 and 750 amu.
RESULTS
Mass spectrometric identification of siderophores produced by P. stutzeri in aerobic cultures.
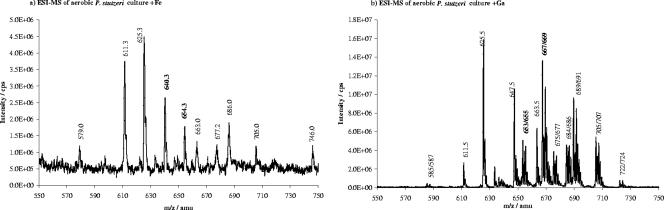
The ferric extracts of aerobic cultures of P. stutzeri produced a number of peaks in the m/z range of 500 to 800 (Fig. 2a), of which 654.3 was the m/z of the proton adduct of ferrioxamine E, which has previously been shown to be produced by P. stutzeri (19). After gallium exchange, Ga-desferrioxamine E was found at m/z 667 and 669 ([M+H]+), 684 and 686 ([M+NH4]+), 689 and 691 ([M+Na]+), and 705 and 707 ([M+K]+) (Fig. 2b). Moreover, the characteristic gallium isotope pattern was recognized at m/z 653 and 655 and at m/z 675 and 677 (Fig. 2b), which corresponds to the proton and sodium adducts, respectively, of the ferric peak observed at m/z 640 (Fig. 2a). The substance giving rise to these peaks was called “unknown 1.” The ammonium and potassium adducts of the m/z 653 and 655 peaks would appear at m/z 670 and 672 and at m/z 691 and 693, respectively; however, these areas were unfortunately overlapped by the peaks at m/z 667 and 669 and at m/z 689 and 691 of Ga-desferrioxamine E. These results indicated that P. stutzeri grown under aerobic conditions produced at least two siderophores, i.e., ferrioxamine E and one siderophore 14 amu lower than the molecular mass of ferrioxamine E. Although two major peaks were found at 611.3 and 625.3 amu in the ferric extract, these substances were shown not to bind to gallium and hence were most likely not siderophores.
FIG. 2.
ESI-MS spectra of extracts of aerobic cultures of P. stutzeri with iron(III) amendment (a) where the peak at 654 amu was ferrioxamine E and the peak at 640 amu was the ferrioxamine E analogue called unknown 1 (D2). After replacement of iron(III) by gallium(III) (b), gallium desferrioxamine produced peaks at m/z 667 and 669 (proton adduct), 684 and 686 (ammonium adduct), 689 and 691 (sodium adduct), and 705 and 707 amu (potassium adduct). The gallium complex of the unknown siderophore called unknown 1 produced peaks at m/z 653 and 655 amu (proton adduct) and 675 and 677 (sodium adduct), while the potential ammonium adduct (expected at 670 and 672 amu) and potassium adduct (expected at 691 and 692 amu) were overlapped with adducts of gallium desferrioxamine E. The peaks at 611 and 625 amu were not affected by replacement of iron with gallium.
Chromatographic separation and identification of siderophores in ferric and gallium-amended extracts of the aerobic culture of P. stutzeri.
Ferric and gallium-amended extracts were analyzed by chromatography using both a C18 column and a PGC column (Fig. 3), onto which gallium and iron complexes of reference siderophores, i.e., desferrioxamine B, G, and E, also were injected for retention time identification. The eluting peaks were detected in scan mode between 550 and 750 amu. When the reference siderophores were separated on the C18 column, the retention times were quite similar for the linear metal-bound desferrioxamines, i.e., both were found at 10 min, while iron and gallium complexes of the cyclic desferrioxamine E eluted later and were observed at 16 min. On the PGC column, the retention time of metal-bound desferrioxamine E was approximately the same as the one found on the C18 column. On the other hand, the metal complexes of the two linear desferrioxamines (B and G) produced broad peaks at 19 and 25 min, respectively, on the PGC column.
FIG. 3.
Extracted ion chromatograms of P. stutzeri cultivated under aerobic conditions with amendment of ferric iron (a) or gallium (b), showing peaks of four siderophores. In the ferric extract (a), the ferric complex of unknown 2 (unk. 2) was detected at 8.6 min and m/z 626 amu, ferrioxamine G (FOG) at 10.7 min and m/z 672 amu, unknown 1 (unk. 1) at 11.8 min and m/z 640 amu, and ferrioxamine E (FOE) at 16.3 min and m/z 654 amu. After replacement of iron with gallium, the same siderophores bound to both 69Ga and 71Ga were detected, i.e., unknown 2 at 7.6 min and m/z 639 and 641 amu, gallium desferrioxamine G at 9.5 min and m/z 685 and 687 amu, unknown 1 at 10.7 min and m/z 653 and 655 amu, and gallium desferrioxamine E at 15.7 min and m/z 667 and 669 amu. For all peaks in panel b, the lowest signal corresponds to the 71Ga isotope while the most intense signal is produced by the 69Ga isotope. Separation conditions: HyPurity C18 column, 100 by 0.5 mm, eluted with 10 μl/min of a mobile phase containing 5% acetonitrile in formiate buffer (pH 4.0), 11 mM.
In the ferric and gallium-amended extracts of aerobic cultures of P. stutzeri, desferrioxamine E was identified as both iron and gallium complexes at 16 to 18 min on the C18 column and the PGC column (Fig. 3), in agreement with the retention times observed for the reference substance. The potential siderophore, called unknown 1, at m/z 640 or at m/z 653 and 655 for the ferric and gallium complexes, respectively, was found at approximately 11 min on C18 (Fig. 3) and at 10 min on PGC. In addition to the results from the MS experiments, minor peaks were found for gallium and iron complexes of desferrioxamine G and, further, one unknown potential siderophore (“unknown 2”) with a mass 28 amu lower than that of ferrioxamine E. The retention time of unknown 2 was 6 to 8 min on both the C18 (Fig. 3) and PGC columns. Desferrioxamine G appeared as both iron and gallium complexes at approximately 9 min using the C18 column (Fig. 3), in agreement with the retention time observed for the reference substance. It was not seen, however, using PGC, which may be due to a combination of a substance at a low concentration producing a broad peak. In addition to the proton adducts of the siderophores, sodium and potassium adducts were observed for both iron(III) and gallium(III) complexes. Furthermore, in the gallium(III)-exchanged extract, the proton adducts of the ferric complexes were observed in addition to those of the gallium complexes. The fact that ferrioxamine G and unknown 2 were not observed by MS is probably due to noise interfering with the signals at low concentrations. Hence, on both columns the retention time order, from least to greatest, is unknown 2, unknown 1, and ferrioxamine E, indicating that the two unknowns are slightly less hydrophobic than ferrioxamine E. The fact that the elution order of the two unknowns, in relation to ferrioxamine E, was not altered with the change from the C18 column to PGC indicates that these are more structurally similar to ferrioxamine E than to ferrioxamine B and G, i.e., the two unknowns are most likely cyclic ferrioxamines. The concentration of ferrioxamine E in the ferric-amended culture of aerobically grown P. stutzeri was in the low micromolar range. Assuming similar mass spectrometric responses for the two unknowns as for ferrioxamine E, the amount of unknown 1 was 38% of that of ferrioxamine E, while that of unknown 2 was 1% of that of ferrioxamine E in the ferric extract. The area ratio in the gallium-amended extract was different from that of the ferric extract. The efficiency of gallium replacement, however, may differ between structurally related siderophores (20).
Partial characterization of siderophores by ESI-MS/MS.
Fragmentation of gallium(III)-bound siderophores in the extract of aerobically grown P. stutzeri was compared with reference spectra of gallium-bound desferrioxamine E and with those of the linear desferrioxamines, i.e., B and G (Table 1). In order to facilitate fragment identification, ferric ferrioxamine complexes of the reference siderophores were also analyzed. No difference in fragmentation patterns was noted between the gallium- and iron-bound ferrioxamines. However, all fragments produced by a neutral loss of 300 amu or less were shown to contain the metal. The main fragments produced by metal-chelated desferrioxamine B were produced by loss of 118, 200, and 217 amu, but minor fragments resulting from loss of 17, 18, 100, 146, and 160 amu were also observed, as shown for Ga-desferrioxamine B (Fig. 4d). The main fragments correspond to an N-terminal neutral loss of NH2—(CH2)5—NH(OH) (−118), NH2—(CH2)5—N(OH)— CO—;CH2—;CH=CO (−200), and NH2—(CH2)5—N(OH)— CO—(CH2)2—CO—NH2 (−217), as noted before by Groenewold et al. (14). C-terminal cleavage produced the neutral fragment resulting from loss of 160 amu, i.e., of CH3—CO— N(OH)—(CH2)5—NH2, also observed by Groenewold et al. (14), resulting in a fragment at m/z 467 in the case of Ga-desferrioxamine B. The fragmentation pattern of Ga-desferrioxamine G showed similarities to that of Ga-desferrioxamine B (Table 1), i.e., the fragments produced by loss of 17, 18, 118, 146, and 200 amu were recognized as in the Ga-ferrioxamine B spectrum. In the Ga-desferrioxamine G spectrum, however, the main fragment was produced by neutral loss of 100 amu, i.e., by loss of C-terminal COOH—CH2—CH=CO. Further C-terminal cleavage of Ga-desferrioxamine G by loss of 218 amu (COOH— (CH2)2—CO—N(OH)—(CH2)5—NH2) resulted in a fragment detected at 467 amu, which is also found in the spectrum of Ga-desferrioxamine B when the part of the molecule differing between ferrioxamine B and G has been cleaved off. The fragmentation pattern of cyclic ferrioxamine E differed from this (Table 1; Fig. 4a). In contrast to the linear ferrioxamines, a number of fragments formed by loss of 35 to 100 amu were seen by loss of 18 (H2O), 36 (2 H2O), 44 (CO2), 46 (HCOOH or H2O plus CO), and 63 (H2O plus CO2 plus NH3) amu. The fragment formed by loss of 100 amu corresponds to loss of C5H12N2.
TABLE 1.
ESI-MS/MS fragmentation of extracted gallium-bound siderophores for P. stutzeri grown under aerobic conditions and for both iron(III)- and gallium-bound reference substances, ferrioxamines B, G, and E, obtained at a collision energy of 40 eV
| Loss of amu | Fragment abundancea
|
||||||
|---|---|---|---|---|---|---|---|
| B ref | G ref | G sample | E ref | E sample | Unknown 1 (D2) | Unknown 2b (X1) | |
| −17 | + | + | + | ||||
| −18 | + | + | + | + | + | + | + |
| −35 | (+) | (+) | (+) | + | + | + | + |
| −36 | (+) | (+) | (+) | + | + | + | + |
| −44 | + | + | + | + | |||
| −46 | (+) | (+) | (+) | + | + | + | + |
| −63 | + | + | + | + | |||
| −86 | + | + | |||||
| −100 | (+) | + | + | + | + | + | + |
| −117 | + | + | + | + | |||
| −118 | + | + | + | ||||
| −134 | + | + | + | + | |||
| −146 | + | + | + | ||||
| −160 | + | ||||||
| −186 | + | + | |||||
| −200 | + | + | + | + | + | + | + |
| −203 | + | ||||||
| −217 | + | + | + | + | + | + | |
| −218 | + | + | |||||
+, major fragment; (+), fragment with low abundance; B ref, G ref, and E ref, ferrioxamine B, G, and E references; G sample and E sample, gallium-bound ferrioxamine G and E samples.
Weak spectra due to low concentration.
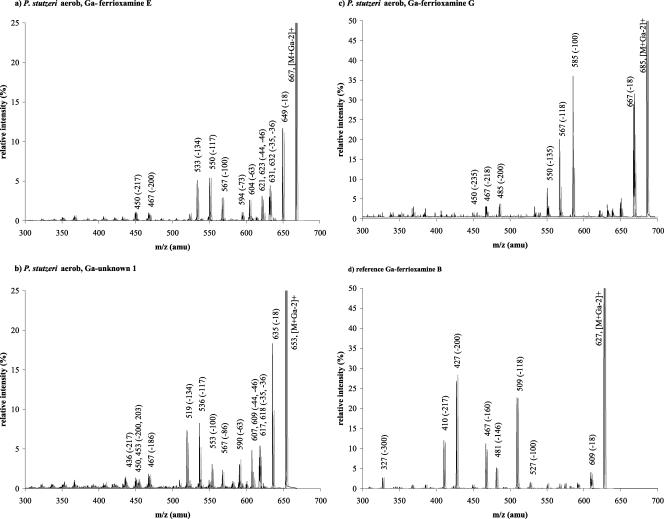
FIG. 4.
ESI-MS/MS spectra of Ga-chelated siderophores from aerobically grown P. stutzeri and reference substances. The fragmentation pattern of the cyclic ferrioxamine Ga-desferrioxamine E (a) differs from those of the linear ferrioxamines Ga-desferrioxamine B (d) and Ga-desferrioxamine G (c). The unknown siderophore (b) fragments in the same way as ferrioxamine E and is thus supposed to be cyclic. Fragmentation of the proton adduct was performed at a collision energy of 40 eV. Black lines indicate the 69Ga isotope, while gray lines indicate the 71Ga isotope. The spectra obtained for Ga-desferrioxamine E and Ga-desferrioxamine G from the aerobic cultures of P. stutzeri were matched by reference substance spectra.
In a comparison of the fragmentation patterns of the siderophores found in the extract of aerobically grown P. stutzeri with the reference spectra, the spectra for Ga-desferrioxamine G (Fig. 4c) and Ga-desferrioxamine E matched the reference spectra and the structures of these two ferrioxamines were verified. The spectra for the two unknown siderophores showed similarities with the fragmentation pattern of Ga-desferrioxamine E, as indicated for unknown 1 (Table 1; Fig. 4b). In addition to the fragments obtained for Ga-ferrioxamine E, three extra fragments were observed, i.e., the losses of 86, 186, and 203 amu. These three fragments are 14 amu smaller than fragments observed for Ga-ferrioxamine E (−100, −200, and −217) and thus contain the part of the siderophore differing between the siderophore unknown 1 and Ga-desferrioxamine E. Since the −100 amu fragment was produced by loss of a diaminopentane residue, the −86 fragment was formed by the loss of a diaminobutane unit. Similarly, the −200 fragment was formed by the loss of succinyl diaminopentane, while a loss of −16 would correspond to succinyl diaminobutane. The loss of −217 amu from Ga-desferrioxamine E occurred through loss of COOH—CH=CH—CO—NH2—(CH2)5—N(OH)H, and thus, the loss of 203 amu would equal the loss of the same fragment with pentane replaced by butane. Thus, the structure of unknown 1 was shown to be the cyclic ferrioxamine D2, as was previously found by Feistner et al. (10). The fragmentation pattern of unknown 2, i.e., the cyclic ferrioxamine 28 amu lower than ferrioxamine E, was similar to the one obtained for unknown 1 (Table 1). Also, in the spectrum of unknown 2, one fragment produced by a loss of 86 amu was observed, and unknown 2 thus seems to contain two butane residues rather than one propane unit.
Evaluation of siderophore production in anaerobically cultured P. stutzeri.
For P. stutzeri grown under anaerobic conditions and analyzed by ESI-MS, no peak with the gallium isotope pattern could be observed in the m/z range of 200 to 800 amu, although the extracted volume was increased from 25 to 100 ml. None of the siderophores produced under aerobic conditions were found in either gallium- or iron-amended extracts. The same result was obtained when the sample was analyzed by LC-ESI-MS. A visual difference between the two culture conditions was noted during the extraction procedure, since the extract of the aerobic culture was pale yellow while the extract of the anaerobically grown P. stutzeri was colorless. Ferric hydroxamates absorb light between 420 and 440 nm and hence give the sample a yellow color (15). In conclusion, no evidence of siderophore production by anaerobically grown P. stutzeri was found.
Analysis of natural water samples by LC-ESI-MS and LC-ESI-MS/MS.
Four natural water samples from the Äspö Hard Rock Laboratory were extracted, and the presence of the three cyclic ferrioxamines shown to be produced by P. stutzeri under aerobic conditions was monitored by chromatographic separation. In order to maintain maximal sensitivity, only ferric extracts were analyzed using the C18 column in combination with on-line preconcentration, lowering the detection limit by a factor of approximately 10 (9). Mass spectrometric detection was performed in the multiple-reaction-monitoring mode. The three cyclic ferrioxamines were searched for using two of the major fragments formed by the loss of 100 and 117 amu, i.e., at m/z 654.4→537.4 and 654.4→554.4 for ferrioxamine E, 640.4→523.4 and 640.3→540.4 for unknown 1, and 626.4→509.3 and 626.4→526.3 for unknown 2. None of the siderophores produced by aerobically cultured P. stutzeri were found in any of the extracts of natural water samples. The lowest detectable concentration in these samples before extraction would be approximately 0.1 nM for the 50-ml samples, while the corresponding limit of detection was approximately 0.02 nM for the 625-ml sample.
DISCUSSION
The ferrioxamines shown to be produced by P. stutzeri under aerobic conditions in this work were characterized by ESI-MS, ESI-MS/MS, and chromatography. In addition to ferrioxamine E, ferrioxamines G and D2, as well as one cyclic ferrioxamine having a molecular mass 28 amu lower than that of ferrioxamine E, were found. According to the fragmentation pattern, the cyclic ferrioxamine with a mass 28 amu lower than that of ferrioxamine E was likely to be the ferrioxamine called X1 by Feistner et al. (10). The chromatographic elution order using the C18 column, from least to greatest, was unknown 2, ferrioxamine G, unknown 1 (ferrioxamine D2), and ferrioxamine E. Comparably, the elution order of ferrioxamines obtained by Feistner et al. (10) on a C18 column, from least to greatest, was ferrioxamine X1, ferrioxamine G, and ferrioxamine D2 < ferrioxamine E. Although different C18 column stationary phases may affect the elution order, the elution order observed in this study was in agreement with the one published by Feistner et al. (10).
The structures of the four ferrioxamines were further elucidated by MS/MS. Ferrioxamines E and G were identified by comparison with spectra obtained for reference substances. Additionally, fragments formed by neutral loss were identified for all four ferrioxamines. Although several other researchers have presented fragmentation pathways for ferrioxamines, most of the work has been done on desferrioxamines or on the linear ferrioxamine B (11, 12, 14, 31). The chelation of a metal was shown to alter the fragmentation of ferrioxamine B (14). Moreover, the fragmentation of cyclic ferrioxamines observed in this work was different from that of linear ferrioxamines, although the fragmentation mechanisms have been suggested to be similar (11). The cyclic ferrioxamines were more stable, i.e., less fragmentation occurred at a given collision energy. Fragmentation of a cyclic ferrioxamine requires two bonds to be broken, which is in contrast to the linear ferrioxamines, where only one bond needs to be broken. Thus, the fragmentation patterns of cyclic ferrioxamines at moderate collision energies were characterized by neutral loss of a number of small fragments, such as H2O and CO2, while the linear ferrioxamines, in addition to the loss of NH3 and H2O, also showed neutral loss of larger fragments. In general, fragmentation often occurred by cleavage at the hydroxamate group and transfer of a hydroxyl unit from the hydroxamate nitrogen to the attached carbonyl, as proposed by Groenewold et al. (14). The structures of ferrioxamines G and E were verified, while the two ferrioxamine E analogues were shown to contain diaminobutane instead of diaminopentane. The two ferrioxamine E analogues are likely to be ferrioxamine D2 and ferrioxamine X1, the latter of which has been identified by Feistner et al. (10). The concentration of ferrioxamines in the ferric amended culture of aerobically grown P. stutzeri was in the lower micromolar range. Although ferrioxamine E was the dominating siderophore, the amount of ferrioxamine D2 was about 38% of that of ferrioxamine E. Bacterial production of ferrioxamines E and G has been shown to be accompanied by production of ferrioxamine E and G analogues having pentane residues replaced by propane or butane (10, 28). In the anaerobic culture of P. stutzeri studied here, no traces of ferrioxamines or any other gallium(III)-chelating compounds were found.
The capability of aerobically and anaerobically grown P. stutzeri to solubilize actinides into the aqueous phase has been ascribed to production of biological ligands. However, the supernatant of aerobically grown P. stutzeri was more effective in maintaining metals in the aqueous phase than the anaerobically produced supernatant (16). Here, the presence of siderophores in the aerobic cultures was indeed verified, while the reason for the observed solubilizing effect provided by anaerobically grown P. stutzeri is still unknown.
The circulations at Äspö Hard Rock Laboratory were set up to investigate how anaerobic, subsurface populations of microorganisms developed as a function of addition of different electron donors, i.e., hydrogen and acetate. Although the main purpose of this experimentation differed significantly from that of the work presented here, it offered an opportunity to investigate the possible production of ferrioxamines and gallium-chelating compounds under unique conditions not available before. The circulating systems were free of metal in contact with the groundwater. It was speculated that the lack of contact with the geological system in the aquifers, with its availability of most elements in the periodic system, including iron, could have induced a production of siderophores in the closed circulations. However, the uncertainty in this speculation was that the anaerobic groundwater show an array of dissolved trace metals, including iron(II), which may have been sufficient for the growth observed. The ferrioxamines produced by P. stutzeri under aerobic conditions were not found in the natural water samples from the Äspö Hard Rock Laboratory, irrespective of the circulation treatment, at concentrations above the detection limits. The detection limits were 0.1 nM and 0.02 nM depending on sample volume. Similarly, no gallium-complexing compound was detected. This suggests that the metal-complexing compounds searched for were not produced under the conditions investigated.
The information gained from the deep groundwater investigation is valuable with respect to the safety analyses of future repositories for spent nuclear fuel. High concentrations of the complexing compounds in question would enhance the transport of several radionuclides because many radionuclides combine with siderophores as discussed in the introduction. Of course, the results here represent only a few of many possible conditions in and around a repository, but the first steps have been taken with respect to method development in the survey of deep groundwater environments for the presence of microbially produced complexing compounds. Future investigations should be expanded to search for complexing compounds produced by fungi in the near and far fields of a repository. Fungi have been found in groundwater (7), and they are present in the bentonite clay to be used as backfill and buffer (27). A larger variety of groundwater than investigated here should also be scanned for complexing compounds, and the array of methods for their detection may need to be expanded.
Conclusions.
In this study, the P. stutzeri strain CCUG 36651 was shown to produce four ferrioxamine siderophores under aerobic growth conditions. Of these, two were shown to be ferrioxamines E and G, while the other two were suggested to be ferrioxamines D2 and X1. In contrast, none of these ferrioxamines produced were found to be produced under anaerobic laboratory conditions, nor were they found in deep groundwater from the Äspö Hard Rock Laboratory. This is, to our knowledge, the first study that has analyzed anaerobic cultures or has investigated anaerobic deep groundwater samples specifically for the presence of siderophores.
Acknowledgments
This project was funded by the Swedish Nuclear Fuel and Waste Management Co., the European Regional Development Fund, and the Faculty of Sciences, Technology and Media at Mid Sweden University.
Footnotes
Published ahead of print on 3 August 2007.
REFERENCES
- 1.Abdallah, M. A. 1991. Pyoverdins and pseudobactins, p. 139-154. In G. Winkelmann (ed.), Handbook of microbial iron chelates. CRC Press Inc., Boca Raton, FL.
- 2.Brainard, J. R., B. A. Strietelmeier, P. H. Smith, and P. J. Langston-Unkefer. 1992. Actinide binding and solubilization by microbial siderophores. Radiochim. Acta 58-59:357-363. [Google Scholar]
- 3.Budzikiewicz, H. 2001. Siderophores of the human pathogenic fluorescent pseudomonads. Curr. Top. Med. Chem. 1:1-16. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty, R. N., H. N. Patel, and S. B. Desai. 1990. Isolation and partial characterization of catechol-type siderophore from Pseudomonas stutzeri RC-7. Curr. Microbiol. 20:283-286. [Google Scholar]
- 5.Crumbliss, A. L. 1991. Aqueous solution equilibrium and kinetic studies of iron siderophore and model siderophore complexes, p. 177-234. In G. Winkelmann (ed.), Handbook of microbial iron chelates. CRC Press Inc., Boca Raton, FL.
- 6.Drechsel, H., and G. Jung. 1998. Peptide siderophores. J. Peptide Sci. 4:147-181. [DOI] [PubMed] [Google Scholar]
- 7.Ekendahl, S., A. H. O'Neill, E. Thomsson, and K. Pedersen. 2003. Characterization of yeasts isolated from deep igneous rock aquifers of the Fennoscandian Shield. Microb. Ecol. 46:416-428. [DOI] [PubMed] [Google Scholar]
- 8.Emery, T. 1986. Exchange of iron by gallium in siderophores. Biochemistry 25:4629-4633. [DOI] [PubMed] [Google Scholar]
- 9.Essén, S. A., D. Bylund, S. J. M. Holmström, M. Moberg, and U. S. Lundström. 2006. Quantification of hydroxamate siderophores in soil solutions of podzolic soil profiles in Sweden. BioMetals 19:269-282. [DOI] [PubMed] [Google Scholar]
- 10.Feistner, G. J., D. C. Stahl, and A. H. Gabrik. 1993. Proferrioxamine siderophores of Erwinia amylovora. A capillary liquid chromatographic/electrospray tandem mass spectrometric study. Org. Mass Spectrom. 28:163-175. [Google Scholar]
- 11.Feistner, G. J., and L. L. Hsieh. 1995. On the collision-activated fragmentation of proferrioxamines: evidence for a succinimide-mediated mechanism. J. Am. Soc. Mass Spectrom. 6:836-846. [DOI] [PubMed] [Google Scholar]
- 12.Gledhill, M. 2001. Electrospray ionization-mass spectrometry of hydroxamate siderophores. Analyst 126:1359-1362. [DOI] [PubMed] [Google Scholar]
- 13.Gledhill, M., P. McCormack, S. Ussher, E. P. Achterberg, R. F. C. Mantoura, and P. J. Worsfold. 2004. Production of siderophore type chelates by mixed bacterioplankton populations in nutrient enriched seawater incubations. Mar. Chem. 88:75-83. [Google Scholar]
- 14.Groenewold, G. S., M. J. van Stipdonk, G. L. Gresham, W. Chien, K. Bulleigh, and A. Howard. 2004. Collision-induced dissociation tandem mass spectrometry of desferrioxamine siderophore complexes from electrospray ionization of UO22+, Fe3+ and Ca2+ solutions. J. Mass Spectrom. 39:752-761. [DOI] [PubMed] [Google Scholar]
- 15.Jalal, M. A. F., and D. van der Helm. 1991. Isolation and spectroscopic identification of fungal siderophores, p. 235-270. In G. Winkelmann (ed.), Handbook of microbial iron chelates. CRC Press, Inc., Boca Raton, FL.
- 16.Johnsson, A., J. Arlinger, A. Ödegaard-Jensen, Y. Albinsson, and K. Pedersen. 2006. Solid-phase partitioning of radionucleides by complexing compounds excreted by subsurface bacteria. Geomicrobiol. J. 23:621-630. [Google Scholar]
- 17.Kalinowski, B. E., A. Oskarsson, Y. Albinsson, J. Arlinger, A. Ödegaard-Jensen, T. Andlid, and K. Pedersen. 2004. Microbial leaching of uranium and other trace elements from shale mine tailings at Ranstad. Geoderma 122:177-194. [Google Scholar]
- 18.Matzanke, B. F. 1991. Structures, coordination chemistry and functions of microbial chelates, p. 15-64. In G. Winkelmann (ed.), Handbook of microbial iron chelates. CRC Press Inc., Boca Raton, FL.
- 19.Meyer, J.-M., and M. A. Abdallah. 1980. The siderochromes of non-fluorescent pseudomonads: production of nocardamine by Pseudomonas stutzeri. J. Gen. Microbiol. 118:125-129. [Google Scholar]
- 20.Moberg, M., E. M. Nilsson, S. J. M. Holmström, U. S. Lundström, J. Pettersson, and K. Markides. 2004. Fingerprinting metal-containing biomolecules after reductive replacement of iron by gallium and subsequent column-switched LC-ICPMS analysis applied on siderophores. Anal. Chem. 76:2618-2622. [DOI] [PubMed] [Google Scholar]
- 21.Neilands, J. B. 1981. Microbial transport compounds (siderophores) as chelating agents, p. 13-31. In A. E. Martell, W. F. Andersson, and D. G. Badman (ed.), Development of iron chelators for clinical use. Elsevier, New York, NY.
- 22.Neilands, J. B. 1984. Siderophores of bacteria and fungi. Microbiol. Sci. 1:9-14. [PubMed] [Google Scholar]
- 23.Neilands, J. B. Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem. 270:26723-26726. [DOI] [PubMed]
- 24.Nielsen. M. E., K. Pedersen, M. Fisk, and J. Istok. 2006. Microbial nitrate respiration of lactate at in situ conditions in groundwater from a granitic aquifer situated 450 underground. Geobiology 4:43-52. [Google Scholar]
- 25.Payne, S. M. 1994. Detection, isolation and characterization of siderophores. Methods Enzymol. 235:329-344. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen, K. 2001. Diversity and activity of microorganisms in deep igneous rock aquifers of the Fennoscandian Shield, p. 97-139. In J. K. Fredrickson, and M. Fletcher (ed.), Subsurface microbiology and biogeochemistry. Wiley-Liss, Inc., New York, NY.
- 27.Pedersen, K. 2002. Microbial processes in the disposal of high level radioactive waste 500 m underground in Fennoscandian shield rocks, p. 279-311. In M. J. Keith-Roach and F. R. Livens (ed.), Interactions of microorganisms with radionuclides. Elsevier, Amsterdam, The Netherlands.
- 28.Reissbrodt, R., W. Rabsch, A. Chapeaurouge, G. Jung, and G. Winkelmann. 1990. Isolation and identification of ferrioxamine G and E in Hafnia alvei. Biol. Metals 3:54-60. [Google Scholar]
- 29.Reitner, J., J. Peckmann, M. Blumenberg, W. Michaelis, A. Reimer, and V. Thiel. 2005. Concretionary methane-seep carbonates and associated microbial communities in Black Sea sediments. Palaeogeogr. Palaeoclimatol. 227:18-30. [Google Scholar]
- 30.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 31.Simionato, A. V. C., G. D. de Souza, E. Rodrigues-Filho, J. Glick, P. Vouros, and E. Carrilho. 2006. Tandem mass spectrometry of coprogen and desferrioxamine hydroxamic siderophores. Rapid Commun. Mass Spectrom. 20:193-196. [DOI] [PubMed] [Google Scholar]
- 32.Whisenhunt, D. W., Jr., M. P. Neu, Z. Hou, J. Xu, D. C. Hoffman, and K. N. Raymond. 1996. Specific sequestering agents for the actinides. 29. Stability of the thorium(IV) complexes of desferrioxamine B (DFO) and three octadentate catecholate or hydroxypyridinonate DFO derivatives: DFOMTA, DFOCAMC, and DFO-1,2-HOPO. Comparative stability of the plutonium(IV) DFOMTA complex. Inorg. Chem. 35:4128-4136. [DOI] [PubMed] [Google Scholar]
- 33.Winkelmann, G. 1992. Structures and functions of fungal siderophores containing hydroxamate and complexone type iron binding ligands. Mycol. Res. 96:529-534. [Google Scholar]
- 34.Winkelmann, G., and H. Drechsel. 1997. Microbial siderophores, p. 200-246. In H.-J. Rehm and G. Reed (ed.), Biotechnology: a multi-volume comprehensive treatise, vol. 7. Products of secondary metabolism. VCH, Weinheim, Germany. [Google Scholar]
- 35.Zawadzka, A. M., R. L. Crawford, and A. J. Paszczynski. 2006. Pyridine-2,6-bis(thiocarboxylic acid) produced by Pseudomonas stutzeri KC reduces chromium(VI) and precipitates mercury, cadmium, lead and arsenic. BioMetals 20:145-158. doi: 10.1007/s10534-006-9022-2. [DOI] [PubMed] [Google Scholar]