Abstract
The fosABCDXE operon encodes components of a putative fructose/mannose phosphoenolpyruvate-dependent phosphotransferase system and a β-fructosidase precursor (FosE) that are involved in the fructooligosaccharide (FOS) utilization pathway of Lactobacillus paracasei 1195. The presence of an N-terminal signal peptide sequence and an LPQAG cell wall anchor motif in the C-terminal region of the deduced FosE precursor amino acid sequence predicted that the enzyme is cell wall associated, indicating that FOS may be hydrolyzed extracellularly. In this study, cell fractionation experiments demonstrated that the FOS hydrolysis activity was present exclusively in the cell wall extract of L. paracasei previously grown on FOS. In contrast, no measurable FOS hydrolysis activity was detected in the cell wall extract from the isogenic fosE mutant. Induction of β-fructosidase activity was observed when cells were grown on FOS, inulin, sucrose, or fructose but not when cells were grown on glucose. A diauxic growth pattern was observed when cells were grown on FOS in the presence of limiting glucose (0.1%). Analysis of the culture supernatant revealed that glucose was consumed first, followed by the longer-chain FOS species. Transcription analysis further showed that the fos operon was expressed only after glucose was depleted in the medium. Expression of fosE in a non-FOS-fermenting strain, Lactobacillus rhamnosus GG, enabled the recombinant strain to metabolize FOS, inulin, sucrose, and levan.
The consumption of fermented food products or dietary supplements containing probiotic species of Lactobacillus and Bifidobacterium has been suggested to promote gastrointestinal (GI) health in humans and other animals by increasing the population of these microorganisms in the GI tract (10, 40). However, the beneficial effects of these bacteria may be transient due to colonization resistance by the commensal microbiota, which restricts the ability of probiotic bacteria to become well established in the intestinal environment (3, 15). An approach to overcome this limitation is to include prebiotics in the host diet. Prebiotics are specific nondigestible dietary sugars that are selectively metabolized by certain probiotic bacteria and that enhance their survival and colonization in the GI tract (12). Such an approach would also enrich the population of indigenous bifidobacteria and lactobacilli, allowing them to occupy a more dominant position in the gut ecosystem.
Fructooligosaccharides (FOS) are among the prebiotic substances that have been shown to selectively stimulate the growth and activity of certain strains of Lactobacillus and Bifidobacterium (4, 11, 13, 16, 47). Two types of FOS, which differ based on their methods of preparation, are commercially available and are widely used in food. One type, referred to as the GFn type of FOS, is enzymatically produced from sucrose and consists of a glucose monomer (G) linked by α-1,2 linkages to two or more β-2,1-linked fructose units (F), forming a mixture of GF2, GF3, and GF4 (16, 17). The other type of commercial FOS is produced by partial enzymatic hydrolysis of the fructan polymer inulin. The resulting product consists of a mixture of linear fructose oligomers, in the FFn form, also linked by β-2,1 linkages and having a degree of polymerization varying from 2 to 10. Due to the presence of a terminal glucose in the inulin molecule, the latter products also contain oligosaccharide species in the GFn form (6).
Despite considerable commercial and research interest in the beneficial effects of FOS, the molecular basis for FOS metabolism by probiotic bacteria and specific members of the intestinal microflora has only recently been examined. It now appears, however, that utilization of FOS occurs via one of two metabolic routes. Either the substrate is transported intact and is hydrolyzed in the cytoplasm, or it is hydrolyzed by extracellular enzymes, followed by subsequent accumulation of the hydrolysis products. In Lactobacillus acidophilus NCFM, for example, the FOS metabolic pathway is encoded by a multiple-sugar metabolism (msm) operon that resembles the msm operon of Streptococcus mutans and the raffinose (raf) operon of Streptococcus pneumoniae (1). The msm operon encodes an ATP-dependent binding cassette-type transport system and a cytoplasmic β-fructosidase that mediate FOS uptake and intracellular hydrolysis. Expression of the operon was induced by sucrose and FOS, but not by glucose or fructose. Similarly, cytoplasmic β-fructofuranosidases from Bifidobacterium adolescentis, Bifidobacterium infantis, and Bifidobacterium lactis have also been reported to hydrolyze FOS (9, 19, 20, 34-36, 46). Although FOS transport in bifidobacteria has not been reported, the presence of at least seven gene loci encoding oligosaccharide transport and metabolism in the genome sequence of Bifidobacterium longum (44) suggests that uptake of FOS may also be mediated by specific oligosaccharide transporters. In contrast, extracellular enzymes that hydrolyze FOS have also been reported for nonintestinal bacteria and include a fructan β-fructosidase from Lactobacillus pentosus and levan biohydrolases from Streptomyces exfoliatus and Microbacterium laevaniformans (39, 41, 45).
Recently, microarray expression analyses of Lactobacillus paracasei 1195 grown on FOS led to the identification of a putative fos operon that plays a major role in the FOS utilization pathway (14). The fosABCDXE operon encodes a putative fructose/mannose phosphotransferase system (PTS) (FosABCDX) and a β-fructosidase precursor (FosE) that has high sequence identity with the putative levanase (lev) operons of Lactobacillus casei strains ATCC 334 and BL23. Inactivation of the fosE gene led to an inability of the mutant strain to grow on FOS and other β-fructose-linked sugars. The deduced amino acid sequence of FosE contains an N-terminal signal peptide sequence and an LPQAG cell wall anchor motif in the C-terminal region, suggesting that FOS may be hydrolyzed extracellularly by FosE, with the subsequent uptake of the hydrolysis products mediated by the FosABCDX PTS. Microarray analyses also indicated that expression of the FOS-induced genes was subject to catabolite regulation by glucose (14). Hence, the objectives of this study were to establish the location of the FOS hydrolysis activity in L. paracasei 1195 and to examine the effect of glucose on FOS utilization by L. paracasei. Additionally, we established the functional role of the fos operon by expressing the fosE gene in Lactobacillus rhamnosus GG, a widely used probiotic strain that has a limited ability to metabolize FOS (21) and other β-fructose-linked carbohydrates.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains and plasmids used in this study are listed in Table 1. The parental strains L. paracasei 1195 and L. rhamnosus GG were routinely grown in MRS broth (Difco, Inc., Ann Arbor, MI) at 37°C in the ambient atmosphere under static conditions, and recombinant strains were grown in MRS medium containing 5 μg/ml of erythromycin (Erm). For growth and enzyme experiments, cells were grown in modified MRS (mMRS) basal medium (14) supplemented with filter-sterilized solutions of FOS of the GFn type (GTC Nutrition, Westminster, CO) or the FFn type (Orafti North America, Malvern, PA), glucose (Sigma-Aldrich, St. Louis, MO), fructose (Sigma), or sucrose (Sigma) at the concentrations indicated below. Inulin- or levan-containing mMRS medium was prepared by addition of inulin (Sigma) or levan (Sigma) to mMRS medium prior to heat sterilization of the culture medium. For diauxie experiments, L. paracasei 1195 was grown in semidefined medium (SDM) (23) containing (per liter) 10 g Bacto Casitone (Difco), 5 g yeast nitrogen base (Difco), 1 g polysorbate 80 (Fisher Chemicals, Fairlawn, NJ), 2 g ammonium citrate (Sigma), 5 g sodium acetate (Sigma), 0.1 g magnesium sulfate (Sigma), 0.05 g manganese sulfate (Sigma), and 2 g dipotassium phosphate (MCB Manufacturing Chemists, Norwood, OH) and supplemented with 0.1% (wt/vol) glucose, 0.35% (wt/vol) FOS (GFn form), or 0.1% glucose plus 0.35% FOS. Escherichia coli DH5α, used as a host for routine cloning procedures, was grown in Luria-Bertani medium or brain heart infusion medium at 37°C with aeration at 200 rpm. When necessary, Erm was added at final concentrations of 250 and 450 μg/ml to brain heart infusion and Luria-Bertani media, respectively.
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Genotype, characteristics, or sequence (5′ to 3′) | Source or reference |
|---|---|---|
| Strains | ||
| L. paracasei | ||
| 1195 | Parent strain, FOS fermenter | UNL collectiona |
| BHe | 1195 isogenic strain with fosE gene disrupted by insertion inactivation | 14 |
| L. rhamnosus | ||
| GG | Parent strain, non-FOS-fermenter | ConAgrab |
| GGE582 | GG harboring pYG582 | This study |
| E. coli DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR endA1 recA1 hsdR17(rK− mK+) phoA supE44 λ−thi-1 gyrA96 relA1 | Gibco-BRLc |
| Plasmids | ||
| pTRKH2 | High-copy-number shuttle cloning vector, P15A ori, pAMβ1 ori, Ermr, lacZ′ | 38 |
| pRH5 | pTRKH2 with fosE gene cloned into XhoI/PstI sites | This study |
| pYG582 | pRH5 with P-GL1 promoter and fosE RBS cloned upstream of fosE | This study |
| Primersd | ||
| fosE-for1 | TGGCTTAGGAAAAGACGCCA | This study |
| fosE-rev1 | TGATCATCAGATACTCGCAA | This study |
| fosE-for2 | CGGACCTCGAGTTGGAAATGGATGAAAAGAAAC | This study |
| fosE-rev2 | ATTATCTGCAGTTAGACTCGCTTCACCCGCCTC | This study |
| PGL1-for | ATCAATGATATCACGGTTTTAAAATGAGCGTTG | This study |
| PGL1-rev | GCTACCTCGAGTCATCCTCCAACTTATTATGTTAATAA | This study |
University of Nebraska Department of Food Science and Technology Culture Collection, Lincoln, NE.
ConAgra Foods Inc., Omaha, NE.
Gibco-BRL, Rockville, MD.
Restriction enzyme sites are underlined, and an RBS is in bold type.
DNA isolation and manipulation.
Isolation of genomic DNA from L. rhamnosus GG was performed as previously described for L. paracasei (14). Plasmid DNA from E. coli was isolated using a Zyppy Plasmid Miniprep I kit (Zymo Research Corp., Orange, CA) according to the manufacturer's recommendations. Restriction enzymes (New England Biolabs Inc., Ipswich, MA, and Takara Mirus Bio Inc., Madison, WI) were used as recommended by the manufacturers. DNA ligation was performed using a Fast-Link DNA ligation kit (Epicenter Biotechnologies, Madison WI) according to supplied instructions. PCR amplicons were generated using the Easy-A high-fidelity PCR cloning enzyme or PfuTurbo DNA polymerase (Stratagene Corp., La Jolla, CA) in an Amplitron II Thermolyne thermocycler (Barnstead/Thermolyne Corp., Dubuque, IA). Primers were synthesized by Sigma-Genosys (The Woodlands, TX). The PCR products were electrophoresed in a 0.8% agarose gel, and DNA fragments were purified using a Zymoclean gel DNA recovery kit (Zymo Research) prior to downstream applications. DNA sequencing was performed by the Genome Core Research Facility (University of Nebraska, Lincoln).
For electroporation, E. coli cells were prepared using protocols of the Wolf Laboratory (http://www.research.umbc.edu/%7Ejwolf/m7.htm). Electroporation was performed in prechilled 0.2-cm electroporation cuvettes (Boca Scientific Inc., Boca Raton, FL) using a Gene Pulser electroporation system (Bio-Rad Laboratories, Inc., Hercules, CA) set at 12.5 kV cm−1, 200 Ω, and 25 μF. Electrotransformation of L. rhamnosus GG was performed as previously described (14). Briefly, stationary-phase cells were used to inoculate 100 ml of MRS broth (2% inoculum) and grown for 3 h (optical density at 625 nm, ∼0.1 to 0.2). Then a freshly prepared filter-sterilized penicillin G solution was added to a final concentration of 10 μg/ml, and the culture was grown for an additional 1.5 to 2.0 h. Cells were harvested by centrifugation at 5,500 × g for 15 min at 4°C and washed with 10 and 50 ml of ice-cold filter-sterilized 1× PEB buffer (272 mM sucrose, 1 mM MgCl2, 7 mM potassium phosphate [KPO4]; pH 7.4) sequentially and then with 10 ml of ice-cold 10% (vol/vol) glycerol; finally, the cells were resuspended in 1 ml of 10% glycerol. For electroporation, ∼0.1 μg of DNA was added to 50 μl of the cells, and the mixture was transferred into a prechilled 0.2-cm electroporation cuvette and incubated on ice for 2 min. Cells were electroporated at 12.5 kV cm−1, 400 Ω, and 25 μF and placed on ice immediately. Transformed cells were supplemented with 950 μl of MRS broth and recovered for 3 to 4 h at 37°C. Cells were then plated onto MRS agar containing 2.5 to 5.0 μg/ml of Erm and incubated at 37°C for 48 to 72 h under ambient atmospheric conditions.
Purification of L. paracasei total RNA.
Total RNA was isolated as previously described (14) using the TRI reagent (Molecular Research Center, Inc., Cincinnati, OH). The RNA samples were subsequently treated with DNase I using a Turbo DNAfree kit (Ambion Inc., Austin, TX). The quality and integrity of RNA samples were assessed spectrophotometrically (A260/A280, 1.6 to 1.9) and gel electrophoresis, as described previously (14).
FOS hydrolysis assay.
L. paracasei 1195 and the BHe mutant strain were grown in mMRS broth containing 1% FOS (GFn form) and harvested by centrifugation at 3,000 × g for 15 min at room temperature when the optical densities at 625 nm reached 0.60 and 0.35, respectively. Culture supernatants were filter sterilized with 0.45-μm filters and concentrated to 1/20 of the initial volume using Amicon Ultra-4 centrifugal filter units (30,000-molecular-weight cutoff; Millipore Corp., Bedford, MA). Cell pellets were washed twice in 0.1 M potassium phosphate buffer (pH 6.6) and resuspended in 1 ml of the same buffer. The cell suspensions were transferred into 1.5-ml conical tubes (BioSpec Products, Inc., Bartlesville, OK) containing 400 mg of 0.1-mm-diameter glass beads (BioSpec Products), and the cells were disrupted by homogenization using a mini beadbeater (BioSpec Products) at 4,200 rpm for six 1-min cycles, with 1 min on ice between cycles. Cell lysates were transferred into fresh tubes, and the fraction containing cell wall fragments was separated from the cytoplasmic extract by centrifugation at 13,800 × g for 10 min at room temperature. The cell wall fraction was resuspended in 1 ml of phosphate buffer, whereas the cytoplasmic extract was concentrated to one-fifth of the initial volume using Amicon Ultra-4 units, as described above.
For β-fructosidase induction experiments, L. paracasei 1195 was subcultured twice in mMRS medium containing 1% FOS (separately for the GFn and FFn types), sucrose, inulin, fructose, glucose, or 0.5% levan. The cultures were subsequently used to inoculate (2% inoculum) 30 ml of mMRS medium containing the sugars at the same concentrations. When the optical density at 625 nm reached 0.6 to 0.7, the cells were collected by centrifugation at 3,000 × g for 20 min at 4°C. Cell fractionation was performed as described above.
For all β-fructosidase assays, 10 μl of a concentrated culture supernatant, cell wall fraction, or cytoplasmic extract was added to 190 μl of a 1% (wt/vol) FOS (GFn or FFn type), sucrose, or inulin solution. The reaction mixtures were incubated at 37°C for 3 h and inactivated by boiling for 2 min, and the activities were expressed as the amount of fructose released per minute per milligram of protein. Fructose concentrations were determined by using a fructose assay kit (Sigma) according to the manufacturer's instructions or by high-performance liquid chromatography using an Aminex HPX-42C column (Bio-Rad Laboratories) and an RI 410 reflective index detector. The internal and external temperatures of the column were maintained at 40 and 85°C, respectively, with a column heater. Water was used as the mobile phase at a flow rate of 0.6 ml/min. Protein concentrations were determined with the Bradford reagent (Sigma), using the manufacturer's specifications. All experiments were done in duplicate.
LDH assay.
Samples of cell-free culture supernatant, cell wall extract, and cytoplasmic extract were assayed for lactate dehydrogenase (LDH) activity as previously described (18). Briefly, the reaction mixtures contained 1 ml of 0.1 M triethanolamine hydrochloride (pH 7.5), 80 μl of 0.1 M sodium pyruvate, 40 μl of 30 mM fructose-1,6-diphosphate, 40 μl of freshly prepared 4 mM NADH, and 40 μl of a cell fraction or the culture supernatant. The decrease in absorbance at 340 nm was recorded over 6 min and used to calculate the LDH activity.
Catabolite repression studies.
Overnight cultures of L. paracasei grown in SDM containing 1% FOS (GFn) were used to inoculate 1.2 liters of SDM containing 0.1% glucose, 0.35% FOS (GFn), or 0.1% glucose plus 0.35% FOS (GFn). Cultures were incubated at 37°C in the ambient atmosphere under static conditions. At various times, the cell densities were recorded, portions of the cultures grown were centrifuged, and the cell supernatants were saved for analysis. In addition, cells grown on SDM containing 0.1% glucose and 0.35% FOS were centrifuged at 3,000 × g for 10 min at room temperature for isolation of total RNA. To prepare RNA samples for gel electrophoresis on a formaldehyde gel, 30 μg of each sample in a 10-μl mixture was mixed with 2.5 μl of 10× morpholinepropanesulfonic acid (MOPS) buffer (0.2 M MOPS, 80 mM sodium acetate, 10 mM EDTA; pH 7.0), 3 μl of a formaldehyde solution (37%, vol/vol; Fisher), 12.5 μl of formamide, and 1 μl of a 1-mg/ml ethidium bromide solution. The mixtures were incubated at 65°C for 10 min, chilled on ice for 2 to 3 min, and then electrophoresed on a formaldehyde gel (1% agarose, 0.66 M formaldehyde, 1× MOPS buffer). The RNA was subsequently transferred onto a Zeta-Probe blotting membrane (Bio-Rad Laboratories) using standard procedures (42). The membrane was then soaked in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 5 min, and RNA was subsequently immobilized on the wet membrane by UV cross-linking twice at 120,000 μJ in a Stratalinker cross-linker (Stratagene). The internal region of the fosE gene (981 bp) used for synthesis of the hybridization probe was amplified from L. paracasei 1195 genomic DNA using the fosE-for1 and fosE-rev1 primers (Table 1) in a 50-μl reaction mixture containing 1 μl of a 10 mM deoxynucleoside triphosphate mixture, 0.5 μg of genomic DNA, 2.5 U of Taq DNA polymerase, and 25 pmol of each primer in 1× Taq DNA polymerase buffer (Stratagene). PCR amplification was carried out using the following conditions: one cycle of 95°C for 3 min, 30 cycles of 30 s at 95°C, 30 s at 55°C, and 1 min at 72°C, and a final cycle of 72°C for 10 min. Synthesis of a digoxigenin-labeled fosE probe with the fosE PCR product, hybridization, and detection of hybridized signals were performed using a DIG High Prime DNA labeling and detection starter kit II (Roche Diagnostics Corp., Indianapolis, IN) as described by manufacturer. Hybridization signals were exposed on X-Omat Blue XB-1 imaging film (Gold Biotechnology, Inc., St. Louis, MO) using multiple exposure times (2 to 8 min) to obtain the optimum signal strength.
Sugar analyses.
Glucose concentrations in culture supernatants were measured using a YSI 2700 SELECT biochemistry analyzer (YSI Inc., Yellow Springs, OH) equipped with glucose membranes (YSI 2365). To determine the concentration of each FOS fraction in the culture supernatants, the samples were spotted along with FOS standards (0.05, 0.1, 0.2, and 0.4%) onto thin-layer chromatography silica gel plates (20 by 20 cm; Whatman Ltd., Kent, United Kingdom). The plates were developed twice in acetic acid-chloroform-water (7:5:1) solvent. Spots were visualized by spraying the plates with ethanolic 50% sulfuric acid and heating them at 115°C for 5 min. The thin-layer chromatography plates were subsequently scanned with an Epson Perfection 1660 photo scanner (Epson America, Inc., Long Beach, CA), and the density of spots on the scanned image was analyzed using the Scion Image for Windows software (http://www.scioncorp.com/frames/fr_download_now.htm).
Expression of the L. paracasei β-fructosidase gene in L. rhamnosus GG.
To introduce the fosE gene into L. rhamnosus GG, a fragment containing the fosE gene with its native ribosomal binding sequence (RBS) and a promoter sequence isolated from L. rhamnosus GG, P-GL1 (33), were sequentially cloned into the pTRKH2 shuttle vector (38). Briefly, the 4,131-bp fosE gene was PCR amplified from the genomic DNA of L. paracasei 1195 using the fosE-for2 and fosE-rev2 primers (Table 1). The fosE amplicon was digested with XhoI and PstI, ligated into pTRKH2 with compatible ends, and transformed into E. coli DH5α. The recombinant plasmid, designated pRH5, was verified by restriction digestion and sequencing. Next, the P-GL1 promoter and the RBS for the fosE gene were cloned upstream of the fosE gene in the pRH5 plasmid. The P-GL1 promoter region was PCR amplified from L. rhamnosus GG genomic DNA using the PGL1-for and PGL1-rev primers (Table 1), with the fosE RBS incorporated into the latter primer. The 103-bp PCR amplicon was restricted with EcoRV and XhoI and ligated into similarly digested pRH5. The ligation products were purified using a DNA Clean & Concentrator-5 kit (Zymo Research) and transformed into E. coli DH5α. Ligation of the PGL-1 promoter and the fosE RBS upstream of the fosE gene in the recombinant plasmid, designated pYG582, was confirmed by DNA sequencing. Recombinant plasmid pYG582 was subsequently electroporated into L. rhamnosus GG, and transformants were recovered on MRS medium plates containing 2 to 5 μg/ml of Erm after incubation at 37°C in the ambient atmosphere for 48 to 72 h. The L. rhamnosus GG transformants harboring pYG582 were streaked on mMRS-1% FOS agar containing 5 μg/ml Erm and 100 mg/liter bromcresol purple to determine their ability to ferment FOS. One recombinant isolate that formed a yellow zone as a result of acid production from fermentation of FOS was selected and designated L. rhamnosus GGE582. The presence of pYG582 in the GGE582 strain was verified by the direct cell PCR method essentially as described previously (5). For phenotypic analysis, strains GG and GGE582 were grown in mMRS and mMRS media containing 5 μg/ml of Erm, respectively, and supplemented with 1% glucose, fructose, sucrose, FOS (both types), inulin, or 0.5% levan.
RESULTS
Location of β-fructosidase activity in L. paracasei 1195.
To identify the location of the β-fructosidase activity in L. paracasei 1195, cells grown in mMRS broth containing 1% FOS (GFn type) were harvested, and three fractions, representing the concentrated culture supernatant, crude cell wall extract, and cytoplasmic extract, were prepared as described above. The same fractions were also obtained from the mutant strain, BHe. Using FOS (GFn type) as the substrate, the β-fructosidase activity of the wild-type strain was detected almost exclusively in the cell wall extract (Table 2). In contrast, the FOS hydrolysis activity in the culture supernatant or the cytoplasmic extract was negligible relative to that in the cell wall extract. No FOS hydrolysis activity was detected in the BHe strain. To confirm that the cell fractionation procedure had adequately separated the different fractions, all cell fractions and supernatants were assayed for LDH, a cytoplasmic marker enzyme. As expected, LDH activity was detected only in the cytoplasmic extract (data not shown).
TABLE 2.
FOS hydrolysis activities of culture supernatants and cell extracts of the L. paracasei 1195 wild-type and BHe mutant strains previously grown on mMRS containing 1% FOS
| L. paracasei strain | Fraction | Amt of fructose released (nmol/min/mg protein) |
|---|---|---|
| 1195 | Culture supernatant | 4 |
| Cell wall extract | 3,400 | |
| Cytoplasmic extract | 13 | |
| BHe | Culture supernatant | 0.2 |
| Cell wall extract | NDa | |
| Cytoplasmic extract | ND |
ND, none detected.
Induction of β-fructosidase activity during growth on various sugars.
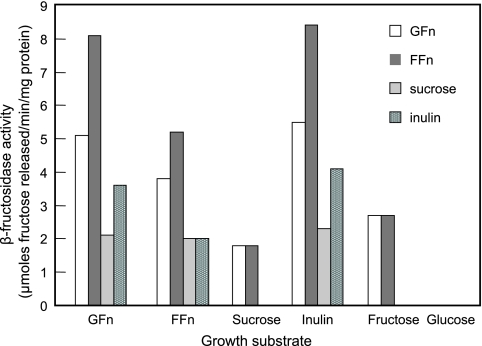
The influence of various carbohydrate growth substrates on the induction of β-fructosidases and their substrate specificities was examined (Fig. 1). Regardless of the carbohydrate source in the media, β-fructosidase activities were present only in the cell wall extracts. Cells grown on inulin exhibited the highest enzyme activities, followed by cells grown on both types of FOS. The two FOS products (GFn and FFn) and inulin also served as the preferred substrates. In contrast, sucrose- and fructose-grown cells had the lowest activities and had activities only when FOS was the substrate. Sucrose was the least preferred substrate, even for sucrose-grown cells. No β-fructosidase activity was detected for the cell wall extract of glucose-grown cells, indicating that the enzyme either was not induced or was repressed in the presence of glucose. Analysis of the FOS (GFn) hydrolysis products by high-performance liquid chromatography showed that fructose and sucrose were the major products from FOS hydrolysis. Inulin hydrolysis generated primarily fructose, and no oligomeric intermediate was released. These observations suggested that the β-fructosidases hydrolyzed the substrates in an exo-type fashion.
FIG. 1.
Induction and substrate specificities of β-fructosidases in cell wall extracts of L. paracasei 1195.
Catabolite repression of FOS utilization by glucose.
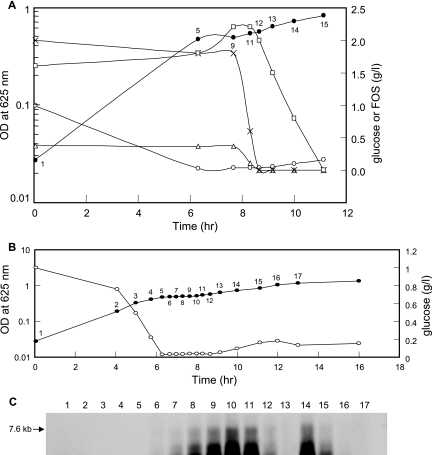
Previous microarray expression analyses suggested that the expression of FOS-induced genes in L. paracasei 1195 was subject to catabolite repression by glucose (14). To further assess the effect of glucose on FOS utilization, the growth of cells in SDM containing both glucose and FOS (0.1 and 0.35%, respectively) was compared to the growth of cells in SDM supplemented with either 0.1% glucose or 0.35% FOS. A typical diauxic growth pattern was observed during growth on glucose plus FOS (Fig. 2). The diauxic lag was likely caused by the depletion of glucose, since cessation of growth was observed at a similar time and cell density for cells grown separately on the same amount of glucose. After the diauxic lag phase, cells resumed growth and entered a second growth phase using FOS as the carbon source, and the cell density of the culture ultimately was approximately the same as the cell density that was observed for cells grown on 0.35% FOS alone (i.e., about 1.5). Sugar analyses of the culture supernatants revealed that FOS was utilized only after glucose was consumed, confirming that glucose was metabolized preferentially (Fig. 3A). When cells entered the second growth phase, GF4 and GF3 were rapidly hydrolyzed, resulting in a transient increase in the GF2 concentration. Subsequently, the GF2 concentration gradually decreased to an undetectable level, and there was a simultaneous increase in the concentrations of glucose and sucrose (data not shown) from the hydrolysis of GF2.
FIG. 2.
Growth of L. paracasei 1195 in SDM supplemented with 0.1% glucose (□), 0.35% FOS (▵), or 0.1% glucose plus 0.35% FOS (•). OD, optical density.
FIG. 3.
Sugar utilization and fos operon expression during diauxic growth of L. paracasei 1195. Cells were grown in SDM containing 0.1% glucose plus 0.35% FOS (A). Cell densities (•) and the concentrations of glucose (○), GF4 (▵), GF3 (×), and GF2 (□) present in the culture supernatant were determined. In a parallel experiment, cells were grown in the same medium (B), and a Northern analysis of the fosABCDXE mRNA transcript levels (C), relative to the cell density (•) and glucose concentration (○) in the culture supernatant, was performed. The numbers on the growth curves in panels A and B correspond to the lane numbers on the Northern blot in panel C and indicate the time points at which cells were collected. OD, optical density.
To examine the kinetics of transcription of the fos operon during the diauxic shift, Northern blot analysis, using a fosE probe, was performed with RNA samples obtained from cells grown on 0.1% glucose plus 0.35% FOS. As expected, no hybridization signal for the fos genes was detected during the first growth phase when glucose was utilized as the preferred carbon source (Fig. 3B and C). Shortly after the onset of the diauxic lag phase, the signal intensity associated with fosE gradually increased, and the maximum transcript levels were observed during the period when GF4 and GF3 were actively hydrolyzed (Fig. 3A). This was followed by a dramatic reduction in the fosABCDXE mRNA level as the GF4 and GF3 were depleted, along with a slight increase in the glucose concentration. A second induction of the fos mRNA transcript was then observed (Fig. 3C, lane 14), coinciding with a decrease in the GF2 concentration. During the next few hours, the signal intensity of the fos operon decreased to an undetectable level (Fig. 3C, lanes 15 to 17). This time frame was associated with the depletion of GF2 and an increase in the glucose and sucrose levels in the culture supernatant.
Expression of the fosE gene in L. rhamnosus GG.
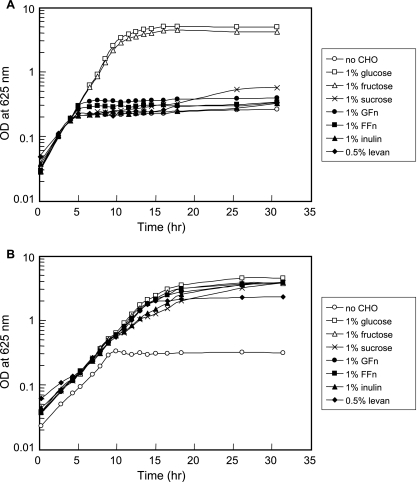
In a previous study it was reported that L. rhamnosus GG, a widely used probiotic strain, was unable to utilize FOS as an energy source (21). However, this strain is able to ferment fructose, indicating the presence of at least one fructose transport system. Thus, only the fosE gene from the fos operon was introduced into the GG strain. To construct a recombinant GG strain capable of metabolizing FOS, the fosE gene, along with its RBS, and the P-GL1 promoter sequence from L. rhamnosus GG (33) were cloned into the pTRKH2 shuttle vector (see Materials and Methods). The resulting construct, pYG582, was transformed into the GG strain. Unlike the parent strain, the recombinant GGE582 strain harboring pYG582 was able to utilize FOS for growth (Fig. 4). In addition, the GGE582 strain was able to grow in mMRS medium containing sucrose, inulin, and levan. None of these sugars supported growth of the parent strain.
FIG. 4.
Growth of the L. rhamnosus GG wild-type strain (A) and the GGE582 recombinant strain (B) in mMRS medium alone (no carbohydrate) or in mMRS medium supplemented with 1% sugar or 0.5% levan, with 5 μg/ml of Erm added to each growth medium for the GGE582 strain. All cultures were inoculated to obtain an initial optical density (OD) at 625 nm of ∼0.02 to 0.05 and were grown at 37°C in the ambient atmosphere under static growth conditions.
DISCUSSION
Recent microarray transcriptome analyses of L. paracasei revealed the presence of a FOS metabolic pathway, encoded by the fosABCDXE operon, that was comprised of a putative cell wall-associated β-fructosidase and a fructose/mannose PTS (14). Expression of the fos genes was induced by FOS and repressed in the presence of glucose. Previous studies of FOS metabolism in L. paracasei 1195, however, had suggested that FOS uptake and hydrolysis were mediated by an ATP-dependent binding cassette transport system and a cytoplasmic β-fructofuranosidase, respectively (22). The cytoplasmic location of the FOS-hydrolyzing enzyme was based in part on the absence of activity in the supernatant and also on the presence of activity associated with the crude cytoplasmic fraction. In this report, the intracellular and cell wall fractions were both examined, and β-fructosidase assays showed that the FOS hydrolysis activity was present primarily in the cell wall extract. This fraction had very high activity and had not previously been assayed for β-fructosidase activity. No cytoplasm-specific LDH activity was detected in the culture supernatant or in the cell wall fraction. These results indicate that cell lysis was minimal when the cultures were harvested prior to cell fractionation and also that the location of the β-fructosidase activity was distinct from the location of the LDH activity. These data provide evidence that FosE is a cell wall-associated β-fructosidase that, like other enzymes possessing LPXTG anchor motifs, faces the extracellular side of the cell wall and therefore catalyzes FOS hydrolysis extracellularly (2, 25). The anchoring of FosE to the cell wall is likely mediated by the action of a sortase that cleaves between the alanyl and glycyl residues of the LPQAG motif and subsequently catalyzes the formation of amide linkage of the alanyl residue to the peptide cross bridge in the peptidoglycan layer (37). The resulting 1,303-amino-acid residue of the mature anchored β-fructosidase thus has an estimated molecular mass of 139 kDa.
The essential role of FosE in the FOS utilization pathway was demonstrated previously, when it was reported that insertional inactivation of the fosE gene severely impaired the ability of the L. paracasei BHe mutant to grow on FOS (14). In the present study, no β-fructosidase activity was detected in the cell wall extract of the BHe mutant. In addition, the mutation prevented utilization of FOS (FFn type), inulin, levan, and sucrose as sole carbon sources, indicating that the fos operon is essential for metabolism not only of FOS but also of other fructose-containing carbohydrates.
Expression of the β-fructosidase was induced during growth on FOS, inulin, and, to a lesser extent, sucrose and fructose but not during growth on glucose. Similarly, the preferred substrates were FOS of the FFn and GFn types, followed by inulin, and there was minor activity with sucrose. These results indicate that this enzyme may have preference for oligosaccharides having β-2,1 linkages. The FFn form of FOS is composed of ca. 75% fructose oligomers which have a degree of polymerization of 2 to 10 and which do not contain a terminal glucose molecule. Thus, most of the FOS chains have more fructosyl units per oligomer as substrates for successive exohydrolysis by β-fructosidase than the GFn form of FOS has. The low activity against the α-1,2 glucose-fructose bond in GFn, as indicated by the near absence of free glucose in reaction mixtures, would also explain why sucrose was not hydrolyzed. Furthermore, the lower activities observed for inulin also indicate a preference for intermediate short-chain oligosaccharides. The exohydrolysis activity of the β-fructosidase is supported by the observation that hydrolysis of the GF4 and GF3 fractions in FOS occurred first, producing GF2, sucrose, and fructose. The latter two compounds then accumulated gradually as the concentration of GF2 decreased. Finally, the fact that no growth was observed on raffinose, a trisaccharide composed of galactose, glucose, and fructose, implies that raffinose is not a substrate for the β-fructosidase (data not shown).
The diauxic growth pattern exhibited by L. paracasei 1195 grown on FOS in the presence of limiting glucose demonstrated that FOS utilization is subject to catabolite repression by glucose. This observation was consistent with the results of transcriptome experiments showing that glucose repressed the transcription of FOS-induced genes (14). During growth on limiting glucose plus FOS, glucose was consumed first, although the cells had been subcultured in medium containing FOS. After the diauxic lag period, FOS were utilized in the order GF4-GF3-GF2, presumably due to the substrate preferences of FosE. Interestingly, Northern hybridization analysis revealed that the expression of the fos genes was not constant during the postdiauxie secondary growth phase. Rather, repression of the fos operon also occurred during the second growth phase. While a small amount of glucose was generated from the hydrolysis of FOS, which may have contributed to the decreased transcript level of the fos mRNA, it also appears that the repression effect was not sufficient to cause a second diauxic lag.
Although the molecular basis of regulation of the fos operon expression was not examined in detail in the present study, given the similarity in operon structure, the transcription of fos in L. paracasei 1195 is likely controlled by similar regulatory mechanisms, as described for the lev operons in L. casei BL23 and Bacillus subtilis (27-32). However, unlike the lev operon of B. subtilis, transcriptional activation of the lev PTS in L. casei BL23 and the fos operon by LevR and FosR, respectively, is independent of a σ54-like sigma factor, since no −12, −24 promoter sequence (CTGGCACN5TTGCA) was found in regions preceding both the BL23 lev operon and the fos operon (7, 8, 32). In BL23, the activity of LevR is regulated by dual PTS-catalyzed phosphorylation at conserved histidine residues in the EIIA and PRD2 domains by P∼His-HPr and P∼His-EIIBLev, respectively (32). In the presence of substrate for Lev-PTS, P∼His-EIIBLev preferably donates its phosphoryl group to the transported sugar, leading to dephosphorylation of LevR at His-776 by P∼His-EIIBLev and LevR activation and thereby induction of the lev PTS. On the other hand, when metabolically preferred PTS sugars, such as glucose, are present, the phosphoryl group of P∼His-HPr is used for sugar phosphorylation. Poor phosphorylation at His-488 by P∼His-HPr renders LevR less active and down regulates expression of the lev PTS. Therefore, the lev operon is subject to carbon catabolite repression by P∼His-HPr dephosphorylation via LevR. The presence of a putative cre sequence overlapping the transcriptional start site of the lev operon of BL23 (32) and the fos operon indicated that the expression of both operons is also controlled by carbon catabolite repression via binding of the catabolite control protein CcpA to the cre site (14, 32). In B. subtilis, accumulation of glycolytic intermediates, such as fructose-1,6-bisphosphosphate, from uptake of rapidly metabolizable sugars was proposed to stimulate the phosphorylation of HPr by HPr kinase at Ser-46 (30). P∼Ser-HPr acts as a corepressor by interacting with CcpA, enabling CcpA to bind to cre, and prevents transcription of the lev operon.
Although certain strains of Lactobacillus are widely used as probiotics due to their various desirable traits (24), their ability to utilize prebiotic oligosaccharides, such as FOS, may be limited (21). We have shown that the introduction of the fosE gene into the non-FOS-fermenting strain L. rhamnosus GG conferred on the recombinant GGE582 strain the ability to utilize not only both forms of FOS efficiently but also other probiotics, such as inulin and levan. Although β-fructosidase activity was not measured in the FOS-fermenting transformant, this strain appeared to grow on these fructans, as well as on glucose and fructose. This demonstrates the feasibility of developing novel probiotic strains having enhanced metabolic functionality.
In contrast to our finding that L. paracasei 1195 could grow on both forms of FOS, Saulnier et al. (43) recently reported that Lactobacillus plantarum WCFS1 was unable to grow on the FFn form. Although L. plantarum WCFS1 also possesses a putative β-fructofuranosidase, this enzyme is apparently intracellular and is part of a sucrose transport and metabolic system. Saulnier et al. suggested that the small GFn oligosaccharides are transported via this sucrose system in L. plantarum WCF1. This strain also had a preference for GF2 and GF3, and there was relatively little consumption of GF4. Although L. paracasei 1195 was originally reported to have a similar substrate preference (21), the current data indicate that all of the FOS fractions, including GF4, were metabolized by this strain.
Another related strain, L. paracasei subsp. paracasei 8700:2, was also reported to use short- and long-chain fractions of FFn FOS simultaneously, although when the organism was grown on inulin and FOS, the FFn chains were preferred (26). Fructose, as well as sucrose and various FFn and GFn oligosaccharides, were also formed during growth on FOS and inulin, indicating that an enzyme capable of extracellular hydrolysis is present in this organism.
Overall, results from this study and a previous mutational analysis of the fosE gene (14) have provided evidence that the fos operon encodes key components for the utilization of FOS and other structurally similar carbohydrates by L. paracasei 1195. While the cell wall-anchored FosE of the fos system may provide versatility for the utilization of larger prebiotic substrates without dependence on dedicated transporters for uptake of the substrates, it may also promote cross-feeding by providing access to the hydrolysis products for other intestinal microorganisms that do not possess a FOS metabolic pathway. In addition, the results show that glucose, generated from hydrolysis of FOS or other glucose-containing polysaccharides, may catabolite repress, at least transiently, FOS metabolism in the GI environment. Collectively, these results emphasize that understanding the mechanisms and regulation of prebiotic sugar utilization by probiotic bacteria and targeted commensals is necessary for rational selection and development of effective probiotics and prebiotics.
Acknowledgments
This study was supported in part by a grant from Dairy Management Inc.
We thank T. Klaenhammer for providing pTRKH2, ConAgra Foods for providing L. rhamnosus GG, and the GTC Nutrition and Orafti Group for their generous donation of commercial prebiotics. We also thank Andy Benson for helpful discussions.
Footnotes
Published ahead of print on 20 July 2007.
REFERENCES
- 1.Barrangou, R., E. Altermann, R. Hutkins, R. Cano, and T. R. Klaenhammer. 2003. Functional and comparative genome analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. Proc. Natl. Acad. Sci. USA 100:8957-8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boekhorst, J., M. W. H. J. de Been, M. Kleerebezem, and R. J. Siezen. 2005. Genome-wide detection and analysis of cell wall-bound proteins with LPxTG-like sorting motifs. J. Bacteriol. 187:4928-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouhnik, Y., P. Pochart, P. Marteau, G. Arlet, I. Goderel, and J. C. Rambaud. 1992. Fecal recovery in humans of viable Bifidobacterium sp. ingested in fermented milk. Gastroenterology 102:875-878. [DOI] [PubMed] [Google Scholar]
- 4.Buddington, R. K., C. H. Williams, S. C. Chen, and S. A. Witherly. 1996. Dietary supplement of neosugar alters the fecal flora and decreases activities of some reductive enzymes in human subjects. Am. J. Clin. Nutr. 63:709-716. [DOI] [PubMed] [Google Scholar]
- 5.Christensen, J. E., and J. L. Steele. 2003. Impaired growth rates in milk of Lactobacillus helveticus peptidase mutants can be overcome by use of amino acid supplements. J. Bacteriol. 185:3297-3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crittenden, R. G. 1999. Prebiotics, p. 141-156. In G. W. Tannock (ed.), Probiotics: a critical review. Horizon Scientific Press, Wymondham, United Kingdom.
- 7.Debarbouille, M., I. Martin-Verstraete, A. Klier, and G. Rapoport. 1991. The transcriptional regulator LevR of Bacillus subtilis has domains homologous to both σ54- and phosphotransferase system-dependent regulators. Proc. Natl. Acad. Sci. USA 88:2212-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debarbouille, M., I. Martin-Verstraete, F. Kunst, and G. Rapoport. 1991. The Bacillus subtilis sigL gene encodes an equivalent of σ54 from Gram-negative bacteria. Proc. Natl. Acad. Sci. USA 88:9092-9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrmann, M. A., M. Korakli, and R. F. Vogel. 2003. Identification of the gene for β-fructofuranosidase of Bifidobacterium lactis DSM10140T and characterization of the enzyme expressed in Escherichia coli. Curr. Microbiol. 46:391-397. [DOI] [PubMed] [Google Scholar]
- 10.Fuller, R., and G. R. Gibson. 1997. Modification of the intestinal microflora using probiotics and prebiotics. Scand. J. Gastroenterol. Suppl.22 2:28-31. [DOI] [PubMed] [Google Scholar]
- 11.Gibson, G. R., E. R. Beatty, X. Wang, and J. H. Cummings. 1995. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108:975-982. [DOI] [PubMed] [Google Scholar]
- 12.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 13.Gibson, G. R., and X. Wang. 1994. Enrichment of bifidobacteria from human gut contents by oligofructose using continuous culture. FEMS Microbiol. Lett. 118:121-127. [DOI] [PubMed] [Google Scholar]
- 14.Goh, Y. J., C. Zhang, A. K. Benson, V. Schlegel, J.-H. Lee, and R. W. Hutkins. 2006. Identification of a putative operon involved in fructooligosaccharide utilization by Lactobacillus paracasei. Appl. Environ. Microbiol. 72:7518-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldin, B. R., S. L. Gorbach, M. Saxelin, S. Barakat, L. Gualtiere, and S. Salminen. 1992. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig. Dis. Sci. 37:121-128. [DOI] [PubMed] [Google Scholar]
- 16.Hidaka, H., T. Eida, T. Takizawa, T. Tokunaga, and Y. Tashiro. 1986. Effects of fructooligosaccharides on intestinal flora and human health. Bifidobacteria Microflora 5:37-50. [Google Scholar]
- 17.Hidaka, H., M. Mirayama, and N. Sumi. 1988. A fructooligosaccharide-producing enzyme from Aspergillus niger ATCC 20611. Agric. Biol. Chem. 52:1181-1187. [Google Scholar]
- 18.Hillier, A. J., and G. R. Jago. 1982. l-Lactate dehydrogenase, FDP-activated, from Streptococcus cremoris. Methods Enzymol. 89:362-367. [DOI] [PubMed] [Google Scholar]
- 19.Imamura, L., K. Hisamitsu, and K. Kobashi. 1994. Purification and characterization of β-fructofuranosidase from Bifidobacterium infantis. Biol. Pharm. Bull. 17:596-602. [DOI] [PubMed] [Google Scholar]
- 20.Janer, C., L. M. Rohr, C. Pelaez, M. Laloi, V. Cleusix, T. Requena, and L. Meile. 2004. Hydrolysis of oligofructose by the recombinant β-fructofuranosidase from Bifidobacterium lactis. Syst. Appl. Microbiol. 27:279-285. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan, H., and R. W. Hutkins. 2000. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl. Environ. Microbiol. 66:2682-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan, H., and R. W. Hutkins. 2003. Metabolism of fructooligosaccharides by Lactobacillus paracasei 1195. Appl. Environ. Microbiol. 69:2217-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimmel, S. A., and R. F. Roberts. 1998. Development of a growth medium suitable for exopolysaccharide production by Lactobacillus delbrueckii spp. bulgaricus RR. Int. J. Food Microbiol. 40:87-92. [DOI] [PubMed] [Google Scholar]
- 24.Kullen, M. J., and T. R. Klaenhammer. 1999. Genetic modification of lactobacilli and bifidobacteria, p. 65-83. In G. W. Tannock (ed.), Probiotics: a critical review. Horizon Scientific Press, Wymondham, United Kingdom.
- 25.Leenhouts, K., G. Buist, and J. Kok. 1999. Anchoring of proteins to lactic acid bacteria. Antonie Leeuwenhoek 76:367-376. [PubMed] [Google Scholar]
- 26.Makras, L., G. Van Acker, and L. De Vuyst. 2005. Lactobacillus paracasei subsp. paracasei 8700:2 degrades inulin-type fructans exhibiting different degrees of polymerization. Appl. Environ. Microbiol. 71:6531-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin, I., M. Debarbouille, A. Klier, and G. Rapoport. 1989. Induction and metabolite regulation of levanase synthesis in Bacillus subtilis. J. Bacteriol. 171:1885-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Verstraete, I., V. Charrier, J. Stulke, A. Galinier, B. Erni, G. Rapoport, and J. Deutscher. 1998. Antagonistic effects of dual PTS-catalysed phosphorylation on the Bacillus subtilis transcriptional activator LevR. Mol. Microbiol. 28:293-303. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Verstraete, I., M. Debarbouille, A. Klier, and G. Rapoport. 1990. Levanase operon of Bacillus subtilis includes a fructose-specific phosphotransferase system regulating the expression of the operon. J. Mol. Biol. 214:657-671. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Verstraete, I., J. Deutscher, and A. Galinier. 1999. Phosphorylation of HPr and Crh by HprK, early steps in the catabolite repression signaling pathway for the Bacillus subtilis levanase operon. J. Bacteriol. 181:2966-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin-Verstraete, I., J. Stulke, A. Klier, and G. Rapoport. 1995. Two different mechanisms mediate catabolite repression of the Bacillus subtilis levanase operon. J. Bacteriol. 177:6919-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maze, A., G. Boel, S. Poncet, I. Mijakovic, Y. Le Breton, A. Benachour, V. Monedero, J. Deutscher, and A. Hartke. 2004. The Lactobacillus casei ptsH147T mutation causes overexpression of a LevR-regulated but RpoN-independent operon encoding a mannose class phosphotransferase system. J. Bacteriol. 186:4543-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCracken, A., M. S. Turner, P. Giffard, L. M. Hafner, and P. Timms. 2000. Analysis of promoter sequences from Lactobacillus and Lactococcus and their activity in several Lactobacillus species. Arch. Microbiol. 173:383-389. [DOI] [PubMed] [Google Scholar]
- 34.Muramatsu, K., S. Onodera, M. Kikuchi, and N. Shiomi. 1992. The production of β-fructofuranosidase from Bifidobacterium spp. Biosci. Biotechnol. Biochem. 56:1451-1454. [Google Scholar]
- 35.Muramatsu, K., S. Onodera, M. Kikuchi, and N. Shiomi. 1993. Purification and some properties of β-fructofuranosidase from Bifidobacterium adolescentis G1. Biosci. Biotechnol. Biochem. 57:1681-1685. [Google Scholar]
- 36.Muramatsu, K., S. Onodera, M. Kikuchi, and N. Shiomi. 1994. Substrate specificity and subsite affinities of β-fructofuranosidase from Bifidobacterium adolescentis G1. Biosci. Biotechnol. Biochem. 58:1642-1645. [Google Scholar]
- 37.Navarre, W. W., and O. Schneewind. 1994. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in Gram-positive bacteria. Mol. Microbiol. 14:115-121. [DOI] [PubMed] [Google Scholar]
- 38.O'Sullivan, D. J., and T. R. Klaenhammer. 1993. High- and low-copy-number Lactococcus shuttle cloning vectors with features for clone screening. Gene 137:227-231. [DOI] [PubMed] [Google Scholar]
- 39.Paludan-Muller, C., L. Gram, and F. P. Rattray. 2002. Purification and characterisation of an extracellular fructan β-fructosidase from a Lactobacillus pentosus strain isolated from fermented fish. Syst. Appl. Microbiol. 25:13-20. [DOI] [PubMed] [Google Scholar]
- 40.Rolfe, R. D. 2000. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 130:396S-402S. [DOI] [PubMed] [Google Scholar]
- 41.Saito, K., K. Kondo, I. Kojima, A. Yokota, and F. Tomita. 2000. Purification and characterization of 2,6-β-d-fructan 6-levanbiohydrolase from Streptomyces exfoliatus F3-2. Appl. Environ. Microbiol. 66:252-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 43.Saulnier, D. M. A., D. Molenaar, W. M. de Vos, G. R. Gibson, and S. Kolida. 2007. Identification of prebiotic fructooligosaccharide metabolism in Lactobacillus plantarum WCFS1 through microarrays. Appl. Environ. Microbiol. 73:1753-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schell, M. A., M. Karmirantzou, B. Snel, D. Vilanova, B. Berger, G. Pessi, M.-C. Zwahlen, F. Desiere, P. Bork, M. Delley, R. D. Pridmore, and F. Arigoni. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 99:14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song, E.-K., H. Kim, H.-K. Sung, and J. Cha. 2002. Cloning and characterization of a levanbiohydrolase from Microbacterium laevaniformans ATCC 15953. Gene 291:45-55. [DOI] [PubMed] [Google Scholar]
- 46.Warchol, M., S. Perrin, J.-P. Grill, and F. Schneider. 2002. Characterization of a purified β-fructosidase from Bifidobacterium infantis ATCC 15697. Lett. Appl. Microbiol. 35:462-467. [DOI] [PubMed] [Google Scholar]
- 47.Williams, C. H., S. A. Witherly, and R. K. Buddington. 1994. Influence of dietary neosugar on selected bacterial groups of the human faecal microbiota. Microb. Ecol. Health Dis. 7:91-97. [Google Scholar]