Abstract
Bacterial iodate (IO3−) reduction is poorly understood largely due to the limited number of available isolates as well as the paucity of information about key enzymes involved in the reaction. In this study, an iodate-reducing bacterium, designated strain SCT, was newly isolated from marine sediment slurry. SCT is phylogenetically closely related to the denitrifying bacterium Pseudomonas stutzeri and reduced 200 μM iodate to iodide (I−) within 12 h in an anaerobic culture containing 10 mM nitrate. The strain did not reduce iodate under the aerobic conditions. An anaerobic washed cell suspension of SCT reduced iodate when the cells were pregrown anaerobically with 10 mM nitrate and 200 μM iodate. However, cells pregrown without iodate did not reduce it. The cells in the former category showed methyl viologen-dependent iodate reductase activity (0.31 U mg−1), which was located predominantly in the periplasmic space. Furthermore, SCT was capable of anaerobic growth with 3 mM iodate as the sole electron acceptor, and the cells showed enhanced activity with respect to iodate reductase (2.46 U mg−1). These results suggest that SCT is a dissimilatory iodate-reducing bacterium and that its iodate reductase is induced by iodate under anaerobic growth conditions.
Iodine is one of the essential trace elements for humans and animals and is known to be a constituent of thyroid hormones. Insufficient iodine in the diet can cause iodine deficiency disorders such as endemic goiter and cretinism (14, 15). From a radioecological viewpoint, on the other hand, long-lived iodine-129 (129I; half life, 1.6 × 107 years) is of great concern, since it is one of the most persistent radionuclides released from nuclear facilities into the environment (3, 17, 23, 27, 30). Given its long half-life, 129I is expected to behave similarly to stable iodine (127I) over long time periods, and it possibly accumulates in the human thyroid gland (38). Therefore, it is important to understand the behavior of iodine in the environment for accurate safety assessments of 129I.
The predominant chemical forms of iodine in the environment are iodate (IO3−; oxidation state, +5) and iodide (I−; oxidation state, −1) (12, 41, 44). One of significant pathways in global iodine cycling is iodate reduction to iodide in oceans. The average concentration of total dissolved iodine in seawater is 0.45 μM (44). Thermodynamically, the concentration ratio between iodate and iodide (IO3−/I−) in oxygenated seawater (at pH 8.1 and pE 12.5) should be 3.2 × 1013, indicating that iodate is the more stable form and that iodide should not be detectable in seawater (31, 45). However, significant quantities of iodide at concentrations of up to 0.3 μM are observed in surface waters (4, 33, 34). It is widely speculated that this apparent disequilibrium is caused by biological reduction of iodate to iodide, and marine microorganisms such as bacteria (7, 8, 36) and phytoplankton (5, 39, 47) may play significant roles in the process. Iodide is also found as the dominant form of iodine in deep oxygenated waters (28), anoxic basins (6, 9, 10, 22, 37, 46, 48), and pore waters of marine sediments (11, 20, 26, 29). In these deep waters, iodide is often highly enriched at concentrations of from several micromolars to more than 1 mM. In addition to abiotic chemical reduction of iodate and microbial remineralization of organic iodine compounds, bacterial iodate reduction is expected to be an important process for maintaining the reduced form of iodine in these environments (8-10, 20, 22).
Until now, only a few studies of bacterial iodate reduction have been conducted. Tsunogai and Sase (36) reported that several laboratory strains of nitrate-reducing bacteria reduced iodate in aerobic cultures. They also found that cell extracts of Escherichia coli, which included nitrate reductase activity, reduced iodate. From these results, they proposed that iodate in seawater is reduced by nitrate reductase of marine organisms (36), although this hypothesis has not been investigated further using bacteria. Desulfovibrio desulfuricans ATCC 29577 (7) and Shewanella oneidensis (formerly S. putrefaciens) MR-4 (8) have been reported to reduce iodate under anaerobic conditions, but the enzymes involved in the reaction were not determined. Despite these early studies, there are still many uncertainties about the physiological and biochemical mechanisms of bacterial iodate reduction. For instance, it is not fully understood whether or not bacterial iodate reduction is a dissimilatory process. In addition, little information is available about key enzymes catalyzing the reduction of iodate. These uncertainties result from the limited availability of bacterial isolates with a capacity for iodate reduction. Here, we describe the isolation and characterization of the iodate-reducing bacterium strain SCT. By use of this strain, iodate reductase activity was determined and compared with other reductase activities under aerobic and anaerobic growth conditions. Furthermore, we attempted to grow strain SCT with iodate as the sole electron acceptor in order to assess whether or not the reduction of iodate is a dissimilatory process.
MATERIALS AND METHODS
Enrichment and isolation of strain SCT.
All incubations were carried out at 30°C throughout this study. Basal medium was used for enrichment of iodate-reducing bacteria. The medium contained (liter−1): NH4Cl (0.54 g), KH2PO4 (0.14 g), MgCl2·6H2O (0.20 g), CaCl2·2H2O (0.15 g), Na2SO4·10H2O (0.14 g), NaCl (20 g), Na2MoO4·2H2O (0.24 g), NaHCO3 (2.5 g), vitamin stock solution (1.0 ml), trace metal stock solution (1.0 ml), and resazurin (1.0 mg). Reducing agents such as cysteine, sodium sulfide, and ascorbate were not added, since these compounds reduce iodate abiotically. The medium contained significant quantities of sodium molybdate, an inhibitor of sulfate-reducing bacteria, since hydrogen sulfide produced by the bacteria also reduces iodate abiotically (7). The medium was sterilized by autoclaving at 121°C for 20 min, and the final pH was 7.0.
In anaerobic incubation, 19 ml of the basal medium was dispensed into a 60-ml serum bottle under an N2-CO2 (80:20) gas stream. The bottle was sealed with a thick butyl rubber stopper and an aluminum cap. After the autoclaving, anoxic sterile stock solutions of iodate (KIO3; Wako Pure Chemical Industries, Japan) and electron donor were added to the medium by use of a sterile syringe and needle. Finally, 1 ml of anoxic sediment slurry was inoculated. The sediment was collected from the upper 3 cm of Sagami Bay, Kanagawa, Japan, and the slurry was prepared by suspending the sediment in the anoxic sterile basal medium at 0.05 g (wet weight) ml−1. The incubation under transition conditions was carried out under conditions that were essentially the same as the anaerobic conditions present in the sealed serum bottle, but replacement of air with N2-CO2 gas in the headspace as well as in the liquid phase was omitted. In the transition incubation, resazurin changed color from blue to pink and finally became colorless, indicating that a shift from aerobic to anaerobic conditions actually occurred. In aerobic incubation, the medium was dispensed into a 100-ml Erlenmeyer flask, and the flask was incubated on a rotary shaker at 140 rpm. In both transition and aerobic incubations, 0.17 g liter−1 of K2HPO4 was also added, but NaHCO3 was omitted from the basal medium.
To isolate iodate-reducing bacteria, the slurry incubated with 200 μM iodate for 18 days under the transition conditions was serially diluted and spread on the basal agar medium containing 15 g liter−1 of agar. After aerobic incubation, 26 colonies were removed randomly, purified, and evaluated for their iodate-reducing capacities under the transition growth conditions. Among these, five isolates reduced 200 μM iodate completely within 2 to 3 days. Approximately 500-bp 16S rRNA gene fragments of these isolates were sequenced and aligned. All of these isolates were affiliated with the genus Pseudomonas, and one of the isolates, strain SCT, was chosen for further studies.
Growth conditions for strain SCT.
Cells of SCT were cultured in the basal medium either aerobically in an Erlenmeyer flask or anaerobically in a sealed glass bottle under conditions of an N2-CO2 atmosphere. Iodate (200 μM), nitrate (10 mM), lactate (10 mM), and Casamino Acids (0.01%) were also added, but NH4Cl, NaCl, Na2MoO4·2H2O, and resazurin were omitted from the basal medium. When potential electron donors and electron acceptors were tested, Bacto-tryptone (0.1%) was added instead of Casamino Acids.
Preparation of washed cell suspension.
Cells grown for 24 h as described above were collected by centrifugation (7,000 × g at 4°C for 10 min). After being washed twice with 10 mM potassium phosphate buffer (pH 7.0), the cells were resuspended in the same buffer to achieve an optical density at 600 nm (OD600) of approximately 0.2 (equivalent to 0.14 mg [dry weight] ml−1). After iodate (200 μM) and lactate (10 mM) were added to the suspension, the cells were incubated either anaerobically under conditions of an N2 atmosphere or aerobically.
Preparation and fractionation of crude cell extracts.
For the preparation of the crude extracts, cells grown anaerobically for 24 h were collected, washed, and resuspended in 10 mM potassium phosphate buffer (pH 7.0) to achieve an OD600 of 20. They were disrupted by sonication (Ohtake ultrasonic disintegrator 5202) at 150 W and 100 kHz for 2 min followed by centrifugation (10,000 × g for 10 min at 4°C) to remove cell debris. The soluble fraction, which contained both cytoplasmic and periplasmic proteins, was separated from the membrane fraction by ultracentrifugation (100,000 × g for 1 h at 4°C). The periplasmic fraction was prepared according to the method of Berks et al. (1). Cells were resuspended in a buffer containing 100 mM Tris-HCl (pH 8.0), 3 mM Na2-EDTA, 0.5 M sucrose, and 25 mg ml−1 lysozyme. After incubation at 30°C for 20 min, the periplasmic fraction was separated from the spheroplasts by centrifugation (30,000 × g for 4 min at 4°C). The disruption of spheroplasts, removal of cell debris, and separation of the cytoplasmic fraction from the membrane fraction were carried out as described above.
Enzyme assays.
The reductase activities were assayed spectrophotometrically in a sealed quartz cuvette at 30°C by monitoring the oxidation of reduced methyl viologen (MV; ɛ578 = 9.7 mM−1·cm−1) as an electron donor. The reaction mixture (0.7 ml) contained 50 mM Tris-HCl (pH 7.0), 0.5 mM MV, an appropriate amount of enzyme, and an electron acceptor. In most cases, the electron acceptors were added at a final concentration of 10 mM. Iodate and periodate (IO4−), however, were added at 1 mM, since 10 mM concentrations of these compounds strongly oxidize the reduced MV abiotically. After the reaction mixture was degassed and sparged with N2 gas, the reaction was started by the addition of a small amount of sodium dithionite to give an absorbance of 1.5 to 2.0. One unit (U) of the reductase activity was defined as the amount of enzyme protein required to oxidize 1 μmol of reduced MV per min. The activities were calculated from initial rates of oxidation of MV, and all measurements were corrected for nonenzymatic oxidation of MV by subtracting the rates observed for control samples in which enzyme was omitted from the reaction mixture. In some cases, reduced benzyl viologen (BV; ɛ600 = 14.8 mM−1·cm−1), NADH, and NADPH were used as the electron donors. Protein concentrations were determined by the method of Bradford (2), with bovine serum albumin as a standard protein. Catalase activity was measured spectrophotometrically by monitoring the rate of disappearance of H2O2 (40).
Analytical techniques.
Iodate concentrations were routinely determined colorimetrically according to the method of Truesdale and Moore (35) with some modifications. First, 2% (wt/vol) sulfamic acid (40 μl) and 2N HCl (20 μl) were added to 400 μl of the culture supernatant. After mixing, 0.1% (wt/vol) potassium iodide (400 μl) was added to yield triiodide (I3−), and finally, 0.1% (wt/vol) soluble starch (400 μl) was added to yield a purple iodine-starch complex, which was immediately measured at 525 nm. The detection limit of this method was approximately 5 μM. Iodide levels were determined by ion chromatography (IC) in combination with inductively coupled plasma mass spectrometry (IC-ICP/MS) as described elsewhere (26). Iodate can also be determined by this method, and the results were very similar to those quantified colorimetrically. The detection limit of this method was approximately 10 nM iodide. The nitrite concentration was determined by diazotizing with sulfanilamide followed by coupling with N-(1-naphthyl)ethylenediamine dihydrochloride (25). The nitrate concentration was determined by the same method after nitrate was reduced to nitrite by hydrazine sulfate (13, 25).
Sequencing of 16S rRNA genes.
Genomic DNA was isolated by the method of Hiraishi (16). The 16S rRNA gene was processed using conditions and reagents that were described previously (19). The obtained 16S rRNA gene sequences were subjected to a BLAST search (http://www.ncbi.nlm.nih.gov/BLAST/) to determine 16S rRNA gene similarities. The retrieved sequences were also aligned using the CLUSTAL W program, version 1.6 (32).
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences determined in this study have been deposited in the DDBJ/EMBL/GenBank databases under accession numbers AB284047 through AB284051.
RESULTS
Isolation of strain SCT.
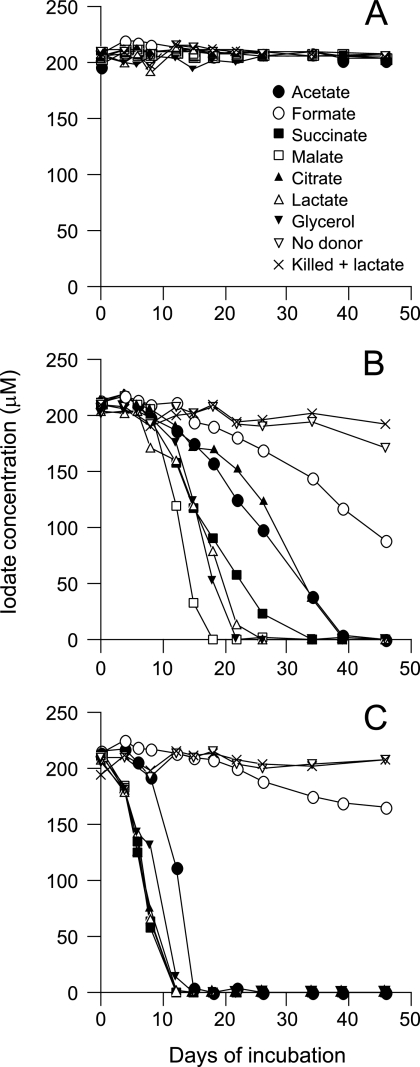
To enrich dissimilatory iodate-reducing bacteria, we first incubated a marine sediment slurry with either 5 or 10 mM iodate under anaerobic conditions. However, no significant reduction was observed even after incubation for 4 months. This was probably due to a toxic effect caused by the high concentrations of iodate (8, 18). Thus, the slurry was then incubated with 200 μM iodate under three types of incubation conditions, i.e., aerobic, anaerobic, and transition conditions. No reduction was observed under the aerobic conditions (Fig. 1A), while complete reduction of iodate occurred within 18 to 39 days under the anaerobic conditions (Fig. 1B). No significant reduction occurred without electron donors or in the autoclaved slurry. Interestingly, much higher rates of iodate reduction were observed under the transition conditions (Fig. 1C), in which the slurry was incubated in sealed serum bottles but without air substitution. Surface seawater collected from Inage beach, Chiba, Japan, was also incubated with iodate in essentially the same way as the sediment slurry, and similar results were obtained (data not shown).
FIG. 1.
Iodate reduction in the sediment slurry incubated under the aerobic (A), anaerobic (B), and transition (C) conditions. The sediment was incubated with iodate at 200 μM and electron donors at 10 mM. The results for samples incubated without electron donors (No donor), and autoclaved samples incubated with lactate (Killed + lactate), are also shown. The experiment was carried out twice, and similar results were obtained. Results from one representative experiment are shown.
An attempt to enrich iodate-reducing bacteria, by transferring the slurry incubated with 200 μM iodate under the anaerobic conditions into the fresh anaerobic medium containing much higher concentrations of iodate (1 to 2 mM), was unsuccessful (data not shown). Since the experimental results described in Fig. 1 implied that the iodate-reducing bacteria were capable of aerobic growth but reduced iodate only under the anaerobic conditions, approximately 30 aerobic bacteria were isolated randomly from the slurry incubated under the transition conditions, and their iodate-reducing capacities were evaluated under the transition growth conditions. As a consequence, we successfully isolated strain SCT, which was able to reduce 200 μM iodate within 3 days. Strain SCT is an aerobic gram-negative bacterium comprising motile, non-spore-forming, short rods. The strain was catalase positive, oxidase positive, and nonfermentative and grew on lactate with nitrate (10 mM) or nitrite (10 mM) as the sole electron acceptor. However, neither 5 mM nor 10 mM iodate could serve as the sole electron acceptor. Analysis of an approximately 1,500-bp 16S rRNA gene sequence revealed that strain SCT was most closely related to Pseudomonas stutzeri DSM5190T (GenBank accession number U26262), with 99.5% sequence similarity. Another related organism was Pseudomonas chloritidismutans DSM13592T (GenBank accession number AY017341; 98.8% sequence similarity), a dissimilatory chlorate (ClO3−)-reducing bacterium.
Iodate reduction by growing cultures and washed cells of strain SCT.
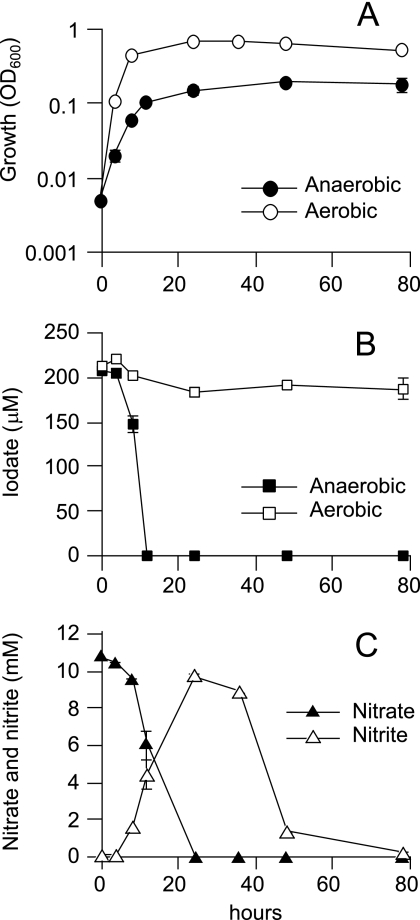
SCT was grown aerobically or anaerobically (under denitrifying conditions) with a 200 μM concentration of iodate (Fig. 2). In the aerobic culture, no significant reduction of iodate was observed. On the other hand, iodate was reduced completely within 12 h in the anaerobic culture. At the end of the cultivation, iodide in the anaerobic culture was quantified by IC-ICP/MS. The result showed that iodide was predominantly produced from iodate at a concentration of 222 μM.
FIG. 2.
Iodate reduction by strain SCT grown under aerobic or anaerobic conditions. Cells were grown in the basal medium containing 10 mM lactate, 10 mM nitrate, and 200 μM iodate. Growth (A), iodate reduction (B), and concentrations of nitrate and nitrite in the anaerobic culture (C) are shown. The error bars indicate standard deviations of the means of the results obtained with triplicate cultures.
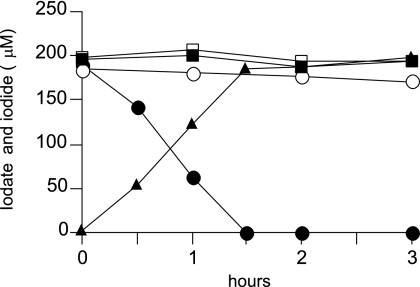
Iodate reduction by a washed cell suspension of SCT was then observed for comparison of the iodate-reducing capacities of aerobically and anaerobically grown cells. We also compared the capacities of the cells grown with and without iodate. As shown in Fig. 3, the cells grown anaerobically without iodate and the aerobically grown cells did not show significant reduction of iodate. However, the cells grown anaerobically with iodate reduced it at a rate of 0.90 μmol h−1 mg dry cells−1. Concomitant production of iodide was also observed. In the absence of an electron donor (lactate), a significant decrease in the rate of reduction (0.12 μmol h−1 mg dry cells−1) was observed (data not shown).
FIG. 3.
Iodate reduction by a washed cell suspension of strain SCT. Cells were pregrown on lactate with (solid symbols) or without (open symbols) 200 μM iodate. After the cultivation, the cells were washed and resuspended in potassium phosphate buffer containing 10 mM lactate and 200 μM iodate. The final cell biomass in each experiment was 2.8 mg (dry weight). Aerobically grown cells were incubated aerobically (squares), and anaerobically grown cells were incubated anaerobically (circles). Iodide production by the cells grown anaerobically with iodate is also shown (solid triangles). The experiment was carried out twice with similar results, and results from one representative experiment are shown. Similar results were also obtained when malate, succinate, acetate, or glycerol was used as the electron donor.
Localization of iodate reductase.
Iodate reductase activity was found in crude extracts of the cells grown anaerobically with iodate (Table 1). MV and BV were suitable electron donors for the detection of iodate reductase, but the activity could not be detected with NADH and NADPH as alternative electron donors. The extracts also showed periodate reductase activity at a level that was similar to the level of iodate reductase activity. The extracts of the cells grown without iodate showed neither iodate nor periodate reductase activity. Nitrate, chlorate, and perchlorate (ClO4−) reductase activities were found in both types of the extracts, but no marked differences were observed in their levels between the cells grown with and without iodate.
TABLE 1.
Reductase activities in the crude extracts of strain SCTa
| Substrate | Electron donor | Growth with IO3− | Growth without IO3− |
|---|---|---|---|
| Iodate | MV | 0.308 ± 0.043 | <0.010 |
| BV | 0.161 ± 0.024 | <0.010 | |
| NADH | <0.030 | <0.030 | |
| NADPH | <0.030 | <0.030 | |
| Nitrate | MV | 4.12 ± 0.249 | 4.17 ± 0.041 |
| BV | 1.92 ± 0.106 | 2.33 ± 0.054 | |
| NADH | 0.080 ± 0.014 | 0.081 ± 0.006 | |
| NADPH | <0.030 | <0.030 | |
| Chlorate | MV | 17.2 | 19.8 |
| Perchlorate | MV | 0.588 | 0.412 |
| Periodate | MV | 0.289 | <0.010 |
| Bromate | MV | <0.010 | <0.010 |
Iodate and nitrate reductase activities are expressed as the means of specific activity (micromoles of electron donors oxidized per minute per milligram of protein) ± standard deviations of the data from at least three determinations. Other reductase activities are expressed as the means of specific activity data from duplicate determinations.
After the ultracentrifugation of the crude extracts, the iodate reductase activity was found almost exclusively in the soluble fraction, while approximately 60% of the total nitrate reductase was detected in the membrane fraction (data not shown). To localize iodate reductase, the periplasmic fraction was prepared from the washed whole cells by lysozyme-EDTA treatment. As shown in Table 2, 76% of the total iodate reductase activity was found in the periplasmic fraction. Catalase (a cytoplasmic marker enzyme) activity was found predominantly in the cytoplasmic fraction, indicating that the periplasmic fraction was prepared appropriately in our experiment.
TABLE 2.
Localization of iodate reductase in cell fractions of strain SCTa
| Strain SCT cell fraction | Total U of iodate reductase (%) | Total U of catalase (%) |
|---|---|---|
| Crude extracts | 7.20 ± 0.94 (100) | NDb |
| Periplasmic | 5.49 ± 0.29 (76.3) | 15.1 ± 11 |
| Spheroplast | 1.61 ± 0.26 (22.4) | 1140 ± 49 |
| Cytoplasmic | 1.26 ± 0.17 (17.5) | 1090 ± 57 |
| Membrane | 0.129 ± 0.087 (1.8) | <10 |
Values are expressed as the means of total activity (U) ± standard deviations of the results obtained in at least three analyses. Iodate reductase activity was determined with MV as the electron donor. Values in parentheses represent percentages of the activity detected in each fraction.
ND, not determined.
Growth of strain SCT with iodate as the sole electron acceptor.
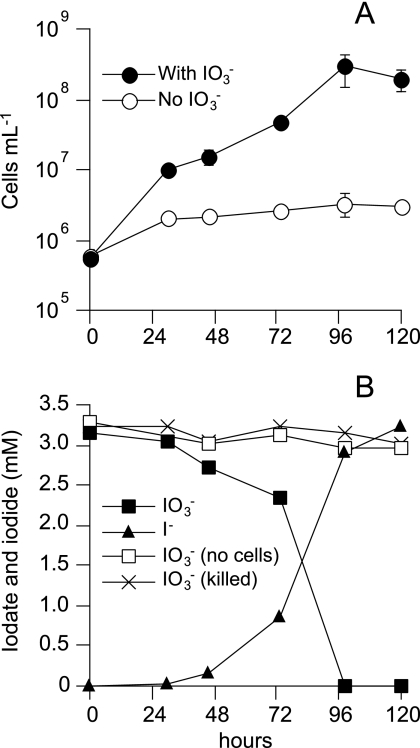
As described above, SCT could not grow with 5 or 10 mM iodate as the sole electron acceptor. However, it showed significant growth (OD600 of 0.1 to 0.15) when much lower concentrations (2, 3, and 4 mM) of iodate were added as the electron acceptor. The growth was nearly proportional to the iodate concentration in the medium. In the presence of 3 mM iodate and 10 mM malate (electron donor), a 520-fold increase in the number of cells was observed with a generation time of 14 h, whereas only a 6-fold increase occurred in the absence of iodate (Fig. 4A). The slight growth in the absence of iodate is probably due to utilization of a trace level of oxygen remaining in the medium, since no reducing agents were added to the medium to prevent abiotic iodate reduction. The growth of SCT coincided with the reduction of iodate, and iodate was transformed completely into iodide (Fig. 4B). No iodate reduction was observed when the medium was not inoculated with live cells or the cells were heat-killed before the inoculation. The anaerobic culture of SCT could be transferred more than five times into the same medium. Malate, lactate, glycerol, glucose, succinate, and acetate could serve as the electron donor, but the growth on acetate was very slow, with a long lag phase of 2 weeks. Formate was not utilized by SCT. No significant growth was observed with 4 mM chlorate, perchlorate, bromate (BrO3−), and periodate as the sole electron acceptor. The cells grown with 3 mM iodate were disrupted, and iodate reductase activity in the crude extracts was determined with MV. The activity (2.46 ± 0.173 U mg protein−1) was eight times higher than that observed in the cells grown with 200 μM iodate (0.308 U mg protein−1), whereas a marked decrease in nitrate reductase activity (1.40 ± 0.134 U mg protein−1) was observed.
FIG. 4.
Growth of strain SCT with iodate as the sole electron acceptor. Cells were grown anaerobically with 10 mM malate as the electron donor and 3 mM iodate as the sole electron acceptor. Bacto-tryptone (0.1%) was also added to the medium. (A) Growth in the presence and absence of iodate. (B) Iodate reduction and concomitant production of iodide. Iodate concentrations in the uninoculated medium (no cells), and in the medium inoculated with heat-killed cells (killed), are also shown. The experiment was carried out twice, and similar results were obtained. Results from one representative experiment are shown.
DISCUSSION
Until now, there have been only a few reports on bacterial iodate reduction. Tsunogai and Sase (36) first reported that several nitrate-reducing bacteria reduce iodate under aerobic conditions and pointed out the importance of nitrate reductase in the reduction of iodate. Washed cells of D. desulfuricans ATCC 29577 (7) and a growing culture of S. oneidensis MR-4 (8) were found to reduce iodate under the anaerobic conditions. In this study, we isolated the iodate-reducing bacterium strain SCT and found that its periplasmic iodate reductase is induced by the presence of iodate under the anaerobic conditions. We also demonstrated that SCT is capable of growing with iodate as the sole electron acceptor. These results suggest that SCT is a dissimilatory iodate-reducing bacterium.
Phylogenetic analysis revealed that SCT is most closely related to P. stutzeri. Since P. stutzeri is generally known as a denitrifying bacterium (21), it is possible that nitrate reductase is involved in the reduction of iodate by SCT, as has been hypothesized by Tsunogai and Sase (36). However, our results clearly indicated that iodate reductase of SCT is induced only when the bacterium is grown with iodate under the anaerobic conditions. By contrast, the levels of nitrate reductase in the cells grown with and without iodate were similar (Table 1). On the basis of these results, we suggest that nitrate reductase of SCT does not play a significant role in iodate reduction. SCT was also closely related to a dissimilatory chlorate-reducing bacterium, P. chloritidismutans. Since iodate (IO3−) is a structural analogue of chlorate (ClO3−) and since SCT showed significant activity with respect to chlorate reductase (Table 1), one may suppose that SCT is a dissimilatory chlorate-reducing bacterium and that it reduces iodate by means of chlorate reductase. However, SCT was not able to grow with chlorate as the sole electron acceptor and it possessed nitrate reductase, both of which were properties distinct from those of P. chloritidismutans (42). In addition, Wolterink et al. (43) showed that chlorate reductase of P. chloritidismutans is a cytoplasmic enzyme and that the enzyme reduces chlorate and bromate but not iodate. These results indicate that iodate reductase of SCT is different from chlorate reductase of P. chloritidismutans. Considering the fact that chlorate-reducing activity is a general feature of membrane-bound respiratory nitrate reductase in many bacteria (24, 50), chlorate reductase activity found in SCT is probably attributable to its nitrate reductase.
Previously, Farrenkopf et al. (8) inoculated 3 × 106 cells ml−1 of S. oneidensis MR-4 into an anaerobic medium containing 250 μM iodate as the sole electron acceptor and found that the number of cells increased to 8 × 106 ml−1 after complete reduction of iodate. Until now, however, there have been no reports of bacterial growth with millimolar concentrations of iodate as the sole electron acceptor. In this study, we found that SCT was capable of growing with 3 mM iodate as the sole electron acceptor and that the number of cells increased from 5.6 × 105 to 2.9 × 108 (Fig. 4). In addition, enhanced activity by iodate reductase was observed in the cells grown with 3 mM iodate. These results strongly suggest that SCT can couple the oxidation of organic carbon to the reduction of iodate and that iodate reductase is an inducible enzyme playing a key role in the reduction of iodate. Considering the high redox potential of iodate reduction (IO3−/I−; E0 = +1.539 V), it would not be surprising if iodate reduction couples to the energy-conserving electron transport pathway in SCT. However, at this stage, details of the energetic properties are not yet clear. Further study is needed to determine the reaction stoichiometry, the type of cytochromes in the iodate-grown cells, and the possible intermediate compounds in the process.
Our results obtained in this study indicate that dissimilatory iodate-reducing bacteria are present in nature. Although iodide is a highly soluble and mobile species, iodate is relatively immobile in the environment because of its sorption on several minerals contained in soils and sediments (49). Thus, bacterial reduction of iodate can affect the mobility of stable iodine (127I) as well as radioactive iodine (129I). Since 129I is one of the most hazardous radionuclides released from reprocessing plants (3, 17, 23, 27, 30) and since it could possibly be leaked from ground storage of nuclear waste, dissimilatory reduction of iodate by bacteria should be considered in predicting the environmental risk of 129I.
Acknowledgments
We thank S. Hattori (Yamagata University) for his kind advice and for providing instructions on reductase assays. We are also grateful to M. Hattori (JAMSTEC) for providing sediment samples, K. Sakamoto (Chiba University) for providing instructions on nitrate determinations, Y. Akiyama (Chiba University) for DNA sequencing, and M. Kishida and Y. Kashiwagi (Gakushuin University) for technical support in IC-ICP/MS analysis.
Footnotes
Published ahead of print on 20 July 2007.
REFERENCES
- 1.Berks, B. C., D. J. Richardson, C. Robinson, A. Reilly, R. T. Aplin, and S. J. Ferguson. 1994. Purification and characterization of the periplasmic nitrate reductase from Thiosphaera pantotropha. Eur. J. Biochem. 220:117-124. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Chem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Buraglio, N., A. Aldahan, G. Possnert, and I. Vintersved. 2001. 129I from the nuclear reprocessing facilities traced in precipitation and runoff in northern Europe. Environ. Sci. Technol. 35:1579-1586. [DOI] [PubMed] [Google Scholar]
- 4.Campos, M. L. A. M., A. M. Farrenkopf, T. D. Jickells, and G. W. Luther III. 1996. A comparison of dissolved iodine cycling at the Bermuda Atlantic Time-series Station and Hawaii Ocean Time-series Station. Deep-Sea Res. Part II Top. Stud. Oceanogr. 43:455-466. [Google Scholar]
- 5.Chance, R., G. Malin, T. Jickells, and A. R. Baker. 2007. Reduction of iodate to iodide by cold water diatom cultures. Mar. Chem. 105:169-180. [Google Scholar]
- 6.Chapman, P. 1983. Changes in iodine speciation in the Benguela Current upwelling system. Deep-Sea Res. 30:1247-1259. [Google Scholar]
- 7.Councell, T. B., E. R. Landa, and D. R. Lovley. 1997. Microbial reduction of iodate. Water Air Soil Pollut. 100:99-106. [Google Scholar]
- 8.Farrenkopf, A. M., M. E. Dollhopf, S. N. Chadhain, G. W. Luther III, and K. H. Nealson. 1997. Reduction of iodate in seawater during Arabian Sea shipboard incubations and in laboratory cultures of the marine bacterium Shewanella putrefaciens strain MR-4. Mar. Chem. 57:347-354. [Google Scholar]
- 9.Farrenkopf, A. M., and G. W. Luther III. 2002. Iodine chemistry reflects productivity and denitrification in the Arabian Sea: evidence for flux of dissolved species from sediments of western India into the OMZ. Deep-Sea Res. Part II Top. Stud. Oceanogr. 49:2303-2318. [Google Scholar]
- 10.Farrenkopf, A. M., G. W. Luther III, V. W. Truesdale, and C. H. van der Weijden. 1997. Sub-surface iodide maxima: evidence for biologically catalyzed redox cycling in Arabian Sea OMZ during the SW intermonsoon. Deep-Sea Res. Part II Top. Stud. Oceanogr. 44:1391-1409. [Google Scholar]
- 11.Fehn, U., G. Snyder, and P. K. Egeberg. 2000. Dating of pore waters with 129I: relevance for the origin of marine gas hydrates. Science 289:2332-2335. [DOI] [PubMed] [Google Scholar]
- 12.Fuge, R., and C. C. Johnson. 1986. The geochemistry of iodine. Environ. Geochem. Health 8:31-54. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, A., K. Sakamoto, and T. Yoshida. 1997. A rapid method for determination of nitrate in soil by hydrazine reduction procedure. Jpn. J. Soil Sci. Plant Nutr. 68:322-326. (In Japanese). [Google Scholar]
- 14.Hetzel, B. S. 1983. Iodine deficiency disorders (IDD) and their eradication. Lancet ii:1126-1129. [DOI] [PubMed] [Google Scholar]
- 15.Hetzel, B. S., and M. T. Mano. 1989. A review of experimental studies of iodine deficiency during fetal development. J. Nutr. 119:145-151. [DOI] [PubMed] [Google Scholar]
- 16.Hiraishi, A. 1992. Direct automated sequencing of 16S rDNA amplified by polymerase chain reaction from bacterial cultures without DNA purification. Lett. Appl. Microbiol. 15:210-213. [DOI] [PubMed] [Google Scholar]
- 17.Hou, X. L., H. Dahlgaard, and S. P. Nielsen. 2000. Iodine-129 time series in Danish, Norwegian and northwest Greenland coast and the Baltic Sea by seaweed. Estuar. Coast. Shelf Sci. 51:571-584. [Google Scholar]
- 18.Johannesson, J. K. 1957. Nature of the bactericidal agent in sea water. Nature 180:285-286. [DOI] [PubMed] [Google Scholar]
- 19.Kanagawa, T., Y. Kamagata, S. Aruga, T. Kohno, M. Horn, and M. Wagner. 2000. Phylogenetic analysis of and oligonucleotide probe development for Eikelboom type 021N filamentous bacteria isolated from bulking activated sludge. Appl. Environ. Microbiol. 66:5043-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy, H. A., and H. Elderfield. 1987. Iodine diagenesis in pelagic deep-sea sediments. Geochim. Cosmochim. Acta 51:2489-2504. [Google Scholar]
- 21.Lalucat, J., A. Bennasar, R. Bosch, E. García-Valdés, and N. J. Palleroni. 2006. Biology of Pseudomonas stutzeri. Microbiol. Mol. Biol. Rev. 70:510-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luther, G. W., III, and T. Campbell. 1991. Iodine speciation in the water column of the Black Sea. Deep-Sea Res. 38:S875-S882. [Google Scholar]
- 23.Moran, J. E., S. Oktay, P. H. Santschi, and D. R. Schink. 1999. Atmospheric dispersal of 129iodine from nuclear fuel reprocessing facilities. Environ. Sci. Technol. 33:2536-2542. [Google Scholar]
- 24.Moreno-Vivián, C., P. Cabello, M. Martínez-Luque, R. Blasco, and F. Castillo. 1999. Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. J. Bacteriol. 181:6573-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullin, J. B., and J. P. Riley. 1955. The spectrophotometric determination of nitrate in natural waters with particular reference to sea-water. Anal. Chim. Acta 12:464-480. [Google Scholar]
- 26.Muramatsu, Y., T. Doi, H. Tomaru, U. Fehn, R. Takeuchi, and R. Matsumoto. 2007. Halogen concentrations in pore waters and sediments of the Nankai Trough, Japan: implications for the origin of gas hydrates. Appl. Geochem. 22:534-556. [Google Scholar]
- 27.Muramatsu, Y., and Y. Ohmomo. 1986. Iodine-129 and iodine-127 in environmental samples collected from Tokaimura/Ibaraki, Japan. Sci. Total Environ. 48:33-43. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama, E., T. Kimoto, K. Isshiki, Y. Sohrin, and S. Okazaki. 1989. Determination and distribution of iodide- and total-iodine in the North Pacific Ocean—by using a new automated electrochemical method. Mar. Chem. 27:105-116. [Google Scholar]
- 29.Pedersen, T. F., and N. B. Price. 1980. The geochemistry of iodine and bromine in sediments of the Panama Basin. J. Mar. Res. 38:397-411. [Google Scholar]
- 30.Raisbeck, G. M., and F. Yiou. 1999. 129I in the oceans: origin and applications. Sci. Total Environ. 238:31-41. [DOI] [PubMed] [Google Scholar]
- 31.Sillen, L. G. 1961. The physical chemistry of seawater, p. 549-581. In M. Sears (ed.), Oceanography. American Association for the Advancement of Science, Washington, DC.
- 32.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian, R. C., J. C. Marty, E. Nicolas, J. Chiavérini, D. Ruiz-Pino, and M. D. Pizay. 1996. Iodine speciation: a potential indicator to evaluate new production versus regenerated production. Deep-Sea Res. Part I Oceanogr. Res. Pap. 43:723-738. [Google Scholar]
- 34.Tian, R. C., and E. Nicolas. 1995. Iodine speciation in the northwest Mediterranean Sea: method and vertical profile. Mar. Chem. 48:151-156. [Google Scholar]
- 35.Truesdale, V. W., and R. M. Moore. 1992. Further studies on the chemical reduction of molecular iodine added to seawater. Mar. Chem. 40:199-213. [Google Scholar]
- 36.Tsunogai, S., and T. Sase. 1969. Formation of iodide-iodine in the ocean. Deep-Sea Res. 16:489-496. [Google Scholar]
- 37.Ullman, W. J., G. W. Luther III, G. J. De Lange, and J. R. W. Woittiez. 1990. Iodine chemistry in deep anoxic basins and overlying waters of the Mediterranean Sea. Mar. Chem. 31:153-170. [Google Scholar]
- 38.Vandecasteele, C. M., M. Van Hees, F. Hardeman, G. Voigt, and B. J. Howard. 2000. The true absorption of 131I, and its transfer to milk in cows given different stable iodine diets. J. Environ. Radioact. 47:301-317. [Google Scholar]
- 39.Waite, T. J., and V. W. Truesdale. 2003. Iodate reduction by Isochrysis galbana is relatively insensitive to de-activation of nitrate reductase activity—are phytoplankton really responsible for iodate reduction in seawater? Mar. Chem. 81:137-148. [Google Scholar]
- 40.Wang, H., Y. Tokushige, H. Shinoyama, T. Fujii, and T. Urakami. 1998. Purification and characterization of a thermostable catalase from culture broth of Thermoascus aurantiacus. J. Ferment. Bioeng. 85:169-173. [Google Scholar]
- 41.Whitehead, D. C. 1984. The distribution and transformations of iodine in the environment. Environ. Int. 10:321-339. [Google Scholar]
- 42.Wolterink, A. F. W. M., A. B. Jonker, S. W. M. Kengen, and A. J. M. Stams. 2002. Pseudomonas chloritidismutans sp. nov., a non-denitrifying, chlorate-reducing bacterium. Int. J. Syst. Evol. Microbiol. 52:2183-2190. [DOI] [PubMed] [Google Scholar]
- 43.Wolterink, A. F. W. M., E. Schiltz, P.-L. Hagedoorn, W. R. Hagen, S. W. M. Kengen, and A. J. M. Stams. 2003. Characterization of the chlorate reductase from Pseudomonas chloritidismutans. J. Bacteriol. 185:3210-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong, G. T. F. 1991. The marine geochemistry of iodine. Rev. Aquat. Sci. 4:45-73. [Google Scholar]
- 45.Wong, G. T. F. 1980. The stability of dissolved inorganic species of iodine in seawater. Mar. Chem. 9:13-24. [Google Scholar]
- 46.Wong, G. T. F., and P. G. Brewer. 1977. The marine chemistry of iodine in anoxic basins. Geochim. Cosmochim. Acta 41:151-159. [Google Scholar]
- 47.Wong, G. T. F., A. U. Piumsomboon, and W. M. Dunstan. 2002. The transformation of iodate to iodide in marine phytoplankton cultures. Mar. Ecol. Prog. Ser. 237:27-39. [Google Scholar]
- 48.Wong, G. T. F., K. Takayanagi, and J. F. Todd. 1985. Dissolved iodine in waters overlying and in the Orca Basin, Gulf of Mexico. Mar. Chem. 17:177-183. [Google Scholar]
- 49.Yamaguchi, N., M. Nakano, H. Taniba, H. Fujikawa, and N. Kihou. 2006. Redox reaction of iodine in paddy soil investigated by field observation and the I K-Edge XANES fingerprinting method. J. Environ. Radioact. 86:212-226. [DOI] [PubMed] [Google Scholar]
- 50.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]