Abstract
The interaction of proteins implicated in dissimilatory metal reduction by Shewanella oneidensis MR-1 (outer membrane [OM] proteins OmcA, MtrB, and MtrC; OM-associated protein MtrA; periplasmic protein CctA; and cytoplasmic membrane protein CymA) were characterized by protein purification, analytical ultracentrifugation, and cross-linking methods. Five of these proteins are heme proteins, OmcA (83 kDa), MtrC (75 kDa), MtrA (32 kDa), CctA (19 kDa), and CymA (21 kDa), and can be visualized after sodium dodecyl sulfate-polyacrylamide gel electrophoresis by heme staining. We show for the first time that MtrC, MtrA, and MtrB form a 198-kDa complex with a 1:1:1 stoichiometry. These proteins copurify through anion-exchange chromatography, and the purified complex has the ability to reduce multiple forms of Fe(III) and Mn(IV). Additionally, MtrA fractionates with the OM through sucrose density gradient ultracentrifugation, and MtrA comigrates with MtrB in native gels. Protein cross-linking of whole cells with 1% formaldehyde show new heme bands of 160, 151, 136, and 59 kDa. Using antibodies to detect each protein separately, heme proteins OmcA and MtrC were shown to cross-link, yielding the 160-kDa band. Consistent with copurification results, MtrB cross-links with MtrA, forming high-molecular-mass bands of approximately 151 and 136 kDa.
Environmental bacteria utilize a wide range of terminal electron acceptors (TEAs) during respiratory metabolism, generally depleting the environment in electron acceptors in a sequential pattern or “redox ladder” starting with acceptors at higher potential followed by those at lower potential (25). For example, facultative anaerobes utilize O2 when present before nitrate, Mn, and Fe species as TEAs. This redox ladder has been attributed to the competition among microorganisms and the energetic benefit of utilizing TEAs yielding higher ΔG per mole. Under anaerobic conditions, microbes can catalyze redox reactions based on >20 elements (25). Of these, six elemental systems are known to be respired in solid form external to the outer membrane or external to the peptidoglycan layer for gram-positive bacteria: S, As, Se, U, Fe, and Mn (9, 13, 23, 27, 37, 40).
Reduction of soluble or insoluble metals as TEAs is referred to as respiratory or dissimilatory metal reduction (DMR) (for a review, see reference 39). Our understanding of DMR is largely derived from studies of the Shewanella genus (22). The Shewanella genus is comprised of gram-negative facultative anaerobes able to utilize a large number of aqueous organic and inorganic metal complexes as well as solid oxides as TEAs. The latter include hydrous ferric oxide, goethite, hematite, and manganese oxides (36, 60), Fe(III), Mn(IV,III), Cr(VI), and U(VI) (1, 10, 20, 31, 36, 37, 61).
The respirome of Shewanella oneidensis is rich in respiratory electron transport proteins, including 42 c-type cytochromes. Previous work has identified some of the potential electron transport proteins involved in DMR (4, 5, 11, 38). However, these genetic studies yield little biochemical information on the sequence of electron transfer from the cytoplasmic membrane (CM), through the periplasm, and across the outer membrane (OM) to the extracellular mineral oxide. At the CM, one source of electrons in lactate-grown cells is formate (51) where oxidation of formate by formate dehydrogenase (FDH) reduces menaquinone to menaquinol. Menaquinol is proposed to reduce CM-localized tetraheme protein CymA. CymA is believed to be the terminal CM electron carrier. Electron transport through the CM results in proton translocation at the level of menaquinone reduction by the proton motive FDH. Reduced CymA passes its electrons to unidentified carriers in the periplasm (e.g., possibly CctA [5]). Electron carriers located in the OM include decaheme proteins OmcA and MtrC. Both of these proteins have been shown to be extracellularly exposed (29). These extracellular heme proteins function to pass the electrons to the external environment (63). MtrB is also essential to DMR and localized in the OM but has no known cofactors (4, 33, 53).
In the work described here, we use protein purification, analytical ultracentrifugation, and formaldehyde cross-linking methods to determine protein-protein interactions of the electron transfer to TEAs operating in Shewanella oneidensis. For the first time, a complex composed of MtrC/MtrA/MtrB is demonstrated to be involved in OM electron transfer. Based upon its location, this complex acts only as a grounding wire and cannot act as part of the proton motive energy-conserving system of the cell which is confined to the inner membrane.
MATERIALS AND METHODS
Organism and growth conditions.
S. oneidensis MR-1 (ATCC 700550) was grown by the method of Myers and Nealson (37) with the following modifications: 30 mM dl-lactate, 4 mM sodium phosphate, and 10 mM HEPES, pH 7.4, with 50 mM ferric citrate in 4-liter flasks under N2 at 30°C. Inoculum was prepared by growth of S. oneidensis in Luria-Bertani (LB) broth (shaken overnight at 21°C) and then centrifuged at 10,000 × g for 10 min at 4°C. The pellet was washed with 0.7% NaCl in 10 mM HEPES, pH 7.4, and then suspended in 1/10 original volume of buffer. Cultures were inoculated with 1 × 106 cells/ml. Anaerobic iron-grown cultures were as previously described (47), using lactate as the carbon source and ferric citrate as the TEA. Cultures were harvested at mid-log phase when the aqueous Fe2+ concentration reached 35 mM as measured with ferrozine (56).
Metal oxides.
Goethite was synthesized by the method of Schwertmann and Cornell (50). Birnessite was synthesized by the method of Lopano et al. (21). The six-line ferrihydrite was synthesized by the method of Guyodo et al. (12). The specific surface area was estimated by the method of Brunauer-Emmett-Teller (BET) (6) for six-line ferrihydrite (174 m2/g) and goethite (30 m2/g).
Membrane isolation and characterization.
The total membrane (TM), CM, and OM fractions were isolated as previously described (47) using a modified version of Myers and Myers (32). These modifications resulted in improved separation, yielding CM and OM with no intermediary fraction. After centrifugation, the OM and CM pellets were stored in 20% glycerol in 10 mM HEPES, pH 7.5, at −80°C. Separation of CM and OM was assessed by NADH oxidase activity assay (41). Protein concentration was measured by the method of Lowry et al. (24).
The periplasm was isolated using osmotic shock of S. oneidensis cultures (16). Cells were suspended in 50 mM Tris-Cl, pH 8.0, 250 mM sucrose, 2.5 mM EDTA for 5 min at room temperature. Cell suspension was centrifuged for 10 min at 14,000 rpm at 4°C. The pellet was suspended in ice-cold 5 mM MgSO4 and kept on ice with occasional swirling. The soluble periplasmic fraction was obtained from the supernatant after centrifugation for 10 min at 14,000 rpm. The spheroplast pellet was suspended in 50 mM Tris-Cl, pH 8.0.
Heterologous expression.
Antibodies were raised to S. oneidensis proteins by using proteins expressed in Escherichia coli as antigens. Genes encoding electron transport proteins were obtained by PCR. Oligonucleotide primers were synthesized (Integrated DNA Technologies) for PCR amplification of mtrC, mtrB, mtrA, cymA, and cctA (Table 1). Chromosomal DNA was isolated from S. oneidensis MR-1 using DNA Easy (QIAGEN). The Taq polymerase-amplified PCR product was subcloned into pGEM-T Easy vector, digested with appropriate restriction endonucleases, and then ligated into pET15b, pET21a, or pET25b (Novagen). E. coli BL21(DE3)/pLysS (Stratagene) cells were used for the expression of MtrC, MtrB, and CctA. MtrA and CctA were expressed in E. coli BL21(DE3)/pEC86 which contained the ccmABCDEFGH genes (cytochrome c maturation) (2).
TABLE 1.
Oligonucleotides used for PCR
| Oligonucleotide | Sequence (5′-3′)a |
|---|---|
| MtrA1 | CATATGAAGAACTGCCTAAAAATGAAAAACCTACTGCC |
| MtrA2 | CTCGAGGCGCTGTAATAGCTTGCCAG |
| MtrC1 | CATATGTTAACCGGCTGTGGTGGAAG |
| MtrC2 | CTCGAGTTACATTTTCACTTTAGTGTGATCTGC |
| MtrB1b | GAATTCATGAAATTTAAACTC |
| MtrB2 | CTCGAGGAGTTTGTAACTCATGCTCA |
| CymA1 | CATATGGTTGTGGGCTATTTTGCAACTCAG |
| CymA2 | CTCGAGTTATCCTTTTGGATAGGGGTGAG |
| CctA1 | CATATGGAAGTCACCCCTGACAAG |
| CctA2 | CTCGAGCTTCTTCAGAACAGACG |
The underlined regions indicate the following restriction endonuclease sites engineered into the oligonucleotides: NdeI sites in set 1 and XhoI sites in set 2 (indicated by 1 and 2, respectively, at the end of oligonucleotide name).
This sequence is for the restriction endonuclease EcoRI.
Cells containing the mtrC-pET21a construct were grown in Luria broth containing 100 μg ampicillin and 34 μg chloramphenicol to an absorbance at 600 nm of 0.6. Isopropyl-β-d-thiogalactopyranoside (IPTG) (1 mM) was then added. Cells were then harvested by centrifugation after incubation for 3 h at 37°C. MtrC was expressed as inclusion bodies and purified (54).
Cells containing the mtrA- and cctA-pET21a construct were heterologously expressed and purified by the method of Pitts et al. (44) with modifications. CctA was purified as described above for MtrA but without detergent. After the polymyxin B sulfate treatment, the cell pellet was homogenized in 20 mM Tris-Cl, pH 8.0, containing 100 mM NaCl and 2% laurylsarcosine. MtrA was most abundant in the detergent-extracted fraction and affinity purified using a Ni-Sepharose column with buffers containing 2% laurylsarcosine.
Cultures containing mtrB-pET25b were grown in LB medium at 37°C to an optical density at 600 nm of 0.6 at 200 rpm. Cultures were then induced to express MtrB by adding 1 mM IPTG and incubating 4 h at 37°C. Cultures were collected by centrifugation, suspended in 20 mM Tris-Cl (pH 8.0) plus 100 mM NaCl, and frozen. MtrB was expressed as inclusion bodies and purified (54).
E. coli BL21(DE3)/pLysS cells containing the cymA-pET15b construct were grown in LB broth containing 50 μg ampicillin and 34 μg chloramphenicol. At an absorbance at 600 nm of 0.6, IPTG (1 mM) was added, and then the cells were incubated for 3 h at 37°C. Cells were collected by centrifugation and then washed with binding buffer (20 mM Tris-Cl, pH 8.0, 100 mM NaCl, and 10 mM imidazole) two times. The pellet was suspended and sonicated three times for 1 min each time. Inclusion bodies were collected by centrifugation at 14,000 × g for 45 min. The pellet was suspended in binding buffer plus 6 M urea and 2% Triton X-100 at room temperature for 30 min. The protein solution was loaded onto a Ni-Sepharose column equilibrated with binding buffer plus 6 M urea. Four column volumes of binding buffer containing 6 M urea were passed through the column. The column was then washed with 3 volumes of wash buffer (20 mM Tris-Cl, pH 8.0, 100 mM NaCl, 50 mM imidazole, and 6 M urea) and eluted with 20 mM Tris-Cl, pH 8.0, 100 mM NaCl, 500 mM imidazole, and 6 M urea. The eluted protein was concentrated and buffer exchanged into 10 mM HEPES, pH 7.5, using Amicon ultrafiltration (10-kDa cutoff).
Antibody production.
Polyclonal antibodies to OmcA, MtrC, MtrA, CymA, and MtrB were raised in New Zealand White rabbits (Covance Custom Immunology Services, Inc.). The immunoglobulin G fraction was further purified by affinity methods using the purified protein antigens on nitrocellulose membranes (26).
Purification of OmcA.
OmcA was purified from anaerobically grown S. oneidensis MR-1 in defined media containing 30 mM lactate and 50 mM ferric citrate (47). The TM fraction was treated with 2% Triton X-100 and loaded onto a Q-Sepharose anion-exchange column and equilibrated with 50 mM HEPES, pH 7.5, and 5% Triton X-100. Fractions were eluted with a gradient of 0 to 0.5 M NaCl over 160 ml at 1 ml/min. Fractions containing OmcA eluted at 30% NaCl as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Coomassie blue and heme stained) were pooled and loaded onto a Mono-Q anion-exchange column. After the column was loaded, it was washed with 50 mM HEPES, pH 7.5, containing 200 mg/ml dodecylmaltoside. OmcA was eluted with a linear gradient of 0 to 0.5 M NaCl. A single band was detected by SDS-PAGE (data not shown). This band was excised, digested with trypsin, and identified by liquid chromatography-tandem mass spectrometry (quadrupole time of flight).
MtrC, MtrA, and MtrB (MtrC/A/B) purification. (i) Anion-exchange chromatography.
TM fractions were isolated (47) and solubilized in 5% Triton X-100 in 50 mM HEPES, pH 7.5. The protein sample was loaded onto a Q-Sepharose (Amersham) column and eluted with a 0 to 0.5 M NaCl gradient (total, 150 ml). Heme proteins were eluted at 32% and 46% NaCl in the gradient.
(ii) Gel permeation chromatography.
Fractions contain MtrC/A/B from the anion-exchange column were purified on a Sephacryl S-300 column (3-cm diameter × 1 m) at room temperature. The buffer was 50 mM HEPES, pH 7.5, containing 250 mM NaCl, and 0.9% N-octylglucoside. The void volume was determined using blue dextran.
Native gel electrophoresis.
Proteins from the TM and the anion-exchange chromatography-purified fractions containing MtrC, MtrA, and MtrB were separated by 5 to 10% gradient native PAGE containing 0.1% Triton X-100 using Tris-glycine buffer, pH 8.8. Gels were stained using Coomassie blue R and heme stain or transferred to nitrocellulose membranes for Western blot analysis.
Sedimentation equilibrium analysis of MtrC/A/B.
Experiments were performed using a Beckman Optima XL-I analytical ultracentrifuge equipped with scanning absorbance optics and an An50Ti rotor. The sample density and the MtrC/A/B partial specific volume of 0.72 ml g−1 were estimated from the amino acid sequence using SEDNTERP software (43). MtrC/A/B samples were diluted to appropriate concentrations with 50 mM HEPES, 200 mM NaCl, 5% (wt/vol) Triton X-100, pH 7.6. A sample volume of 100 μl was loaded into charcoal-filled Epon double-sector cells fitted with quartz windows. Reference sectors were filled with 110 μl buffer. Sedimentation equilibrium experiments were performed at 20°C using speeds of 6,000, 8,000, and 10,000 rpm. Concentration profiles were measured at absorbance wavelengths of 530 nm (2.5 μM) and 430 nm (0.25 μM) for each MtrC/A/B sample. MtrC/A/B sample concentrations, prior to analysis, were estimated based on experimentally derived Soret band extinction coefficients for MtrA (1,320 mM−1 cm−1) (44) and MtrC (1,260 mM−1 cm−1) (R. Hartshorne, B. Jepson, T. Clarke, S. Field, J. Fredrickson, J. Zachara, L. Shi, J. Butt, and D. Richardson, unpublished) and on the assumption of 1:1:1 MtrC:MtrA:MtrB stoichiometry (as suggested from SDS-PAGE analysis…). Scans were recorded every 4 hours, and equilibrium was considered to have been reached when the absorbance values remained constant over a 4-h period. Once equilibrium was attained, five scans were recorded for each sample. The program ULTRASCAN 8.0 (http://www.ultrascan.uthscsa.edu/) was used to simultaneously fit the obtained sedimentation equilibrium profiles obtained at the three different speeds to a single noninteracting species. The solution molecular mass of the MtrC/A/B complex (plus adsorbed detergent) was determined by fitting the absorbance data of the equilibration runs using the exponential equation:
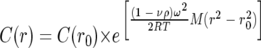 |
where C(r) is the concentration at radius r, C(r0) is the concentration at reference radius r0, M is the solution molecular mass, R is the universal gas constant, T is the temperature, ω is the angular velocity, ρ is the density of the solution, and ν is the partial specific volume of the protein complex. Rearranging this equation gives:
 |
For visualizing the data graphically, measured absorbance can be substituted for concentration and plots of ln absorbance versus (r2 − r02)/2 for a single macromolecular species are expected to give a straight line, the slope of which is proportional to the solution molecular mass.
Kinetics of MtrC/A/B oxidation with various Fe and Mn forms.
Samples of purified MtrC/A/B were chemically reduced with dithionite. Concentrated dithionite was added at small intervals until no further increase was observed at 550 nm. To this solution, ferric citrate, six-line ferrihydrite, goethite, and birnessite were added as described in the figure legends. Heme oxidation was monitored by UV/visible spectroscopy.
Formaldehyde cross-linking.
S. oneidensis MR-1 cells were grown to mid-log phase in modified M1 medium (47) with 30 mM lactate and 50 mM ferric citrate as the electron donor and acceptor, respectively. The cross-linking was performed by the method of Higgs et al. (14). Cells were centrifuged at 16,000 × g for 1 min, then washed with an equal volume of phosphate-buffered saline (PBS), pH 6.8, and suspended in phosphate-buffered saline to an optical density at 550 nm of 0.4. Cells were cross-linked for 0, 30, 60, and 120 min at room temperature with 1% formaldehyde. Cross-linked cells were centrifuged 16,000 × g for 1 min, and the pellet was suspended in 50 μl of 1× denatured gel sample buffer (18) for 5 min at 60°C. Control samples were boiled at 100°C for 10 min for 0- and 120-min samples. A total of 0.1 A550 unit was loaded per well (12.5 μl) on a 6 to 13% gradient SDS-PAGE. Proteins were then transferred to nitrocellulose membranes, and the blots were visualized with the Immobilon Western AP substrate (Millipore) according to the manufacturer's protocol. Molecular masses were determined using Benchmark protein standards (Invitrogen, Inc.). Prior to visualization with antibodies or by heme staining, each blot was first visualized for protein (including molecular mass standards) using Ponceau S. Heme proteins were detected by heme staining with West Pico chemiluminescent substrate (Pierce) by incubating the membrane for 5 min in a 1:1 ratio of the peroxide and chemiluminescence solutions. The membrane was then exposed to CL film (Pierce) for imaging.
RESULTS
Demonstration of antibody specificity.
To monitor the interaction of selected proteins thought to be involved in DMR, we obtained antibodies to these proteins. Polyclonal antibodies were raised in rabbits against purified OmcA, MtrC, MtrB, MtrA, CctA, and CymA. The antibodies were affinity purified, and their specificity was examined with Western blots. Our previous work had shown the specificity of the antibodies to OmcA, MtrC, MtrB, MtrA, and CymA (47). In the present paper, we show the specificity of the antibody to CctA. Separate SDS-polyacrylamide gels were loaded with the same whole-cell lysate of S. oneidensis grown with ferric citrate as the TEA. The proteins were then transferred to a nitrocellulose membrane, and the blot was visualized with purified antibody to CctA (Fig. 1). Only a single cross-reacting band was detected, demonstrating the high degree of specificity of the antibody to CctA.
FIG. 1.

Western blot of whole-cell extracts from S. oneidensis MR-1 using affinity-purified antibodies to CctA. Whole cells were sonicated, and 8-μg samples of proteins was loaded onto 12% SDS-polyacrylamide gels. The positions of molecular mass markers (in kilodaltons) are indicated to the left of the gel.
Affinity of MtrC, MtrA, and MtrB.
In purification of OM heme proteins, we found OmcA to be easily purified (see below) but MtrC to be difficult to purify. Not only was MtrC unstable to purify but it was also difficult to purify away from MtrB. Using antibodies to MtrC, MtrA, and MtrB, we discovered that these three proteins copurify as a complex (Fig. 2). The TM fraction was detergent solubilized with 2% Triton X-100 and then subjected to anion-exchange chromatography on Q-Sepharose. Monitoring heme absorbance at 410 nm, the elution profile showed two major peaks (Fig. 2A). The fractions were then subjected to SDS-PAGE, and the proteins were visualized either by heme staining or by Western blotting. The heme-stained gels showed that the first heme peak off the Q-Sepharose column had a molecular mass of 83 kDa, identical to that of OmcA (data not shown). The second heme peak from the Q-Sepharose column contained two heme bands on the SDS-polyacrylamide gel, and their molecular weights were identical to those of MtrC and MtrA (data not shown). SDS-PAGE and Western blot analysis showed that the first heme peak was OmcA (Fig. 2B). The second heme peak contained MtrC, MtrA, and MtrB (MtrC/A/B). Despite different pI values, these three proteins coeluted from the anion-exchange column. Little or no difference was observed in the distribution of MtrC and MtrB in the fractions. Some MtrA eluted from the column slightly before MtrC and MtrB, suggesting its interaction with MtrB and MtrC to be slightly weaker and that it merely associates with the OM. Nevertheless, the majority of MtrA coeluted with these two proteins.
FIG. 2.
(A) Q-Sepharose anion-exchange chromatography profile of TM fractions from S. oneidensis MR-1 monitored at heme-associated absorbance at 410 nm. (B) Western blots of protein fractions from Q-Sepharose column indicating the elution profiles of OmcA, MtrC, MtrB, and MtrA. Four replicate gels containing 12-μl amounts of protein from fractions 26 to 60 (even-numbered fractions correspond to those labeled on the elution profile) were run on SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Detection of antibodies used Immobilon Western AP substrate. Note that fraction 50 for the MtrC visualization is lower in intensity due to leakage of the well.
The fractions containing MtrC/A/B (fractions 42 to 54) were pooled, concentration by Amicon ultrafiltration (10-kDa cutoff), and then subjected to size exclusion chromatography on Sephacryl S-300 column using 0.9% N-octylglucoside as the detergent. A major heme peak eluted after the void volume (Fig. 3A) and was analyzed by SDS-PAGE (Fig. 3B). Coomassie blue staining showed two major proteins which corresponded to the molecular masses for MtrA and MtrC. These two major bands were shown to be heme proteins as demonstrated by heme staining (Fig. 3B, lane 2). Mass spectroscopy showed that the protein band just above MtrC is MtrB, and in fact, the two were not well resolved by SDS-PAGE. The presence of MtrC, MtrA, and MtrB in the major heme peak was also confirmed by Western blotting with the respective antibodies.
FIG. 3.
Gel permeation chromatography of the MtrC/A/B peak fractions and analysis by SDS-PAGE. Fractions 38 to 56 shown in Fig. 2 were pooled, concentrated, and applied to a gel filtration column. (A) Elution profile of MtrC/A/B from gel filtration (Sephacryl S-300 HR resin; Amersham Biosciences) as monitored from the heme absorbance at 409 nm. The arrow indicates the void volume (blue dextran). (B) 10% SDS-PAGE of MtrC/A/B fraction taken from the peak fraction after gel filtration and visualized with either silver staining (lane 1) or heme staining (lane 2). The predominant heme peaks correspond to MtrC (69 kDa) and MtrA (30 kDa). The positions of molecular mass markers (in kilodaltons) are indicated to the left of the gel.
Native gel electrophoresis of the MtrC/A/B complex.
After chromatography on Q-Sepharose, the MtrC/A/B complex was subjected to native gel electrophoresis which separates proteins based on both size and charge (Fig. 4A). Fractions 38 to 56 (shown in Fig. 2) were pooled, and 15-μl samples were loaded onto three adjacent lanes of the native gel. After electrophoresis, the gel was Western transferred to a nitrocellulose membrane. One of the lanes was visualized with anti-MtrC antibody, another with anti-MtrB antibody, and the third was visualized with anti-MtrA antibody. The nitrocellulose strips were then reassembled in silico to reconstruct the original blot (Fig. 4A). MtrC in lane 1 in Fig. 4A was detected in three bands. As shown by the Western blots in the adjacent lanes, two of the bands near the top of the gel share the same electrophoretic mobility as MtrB and MtrA. This is consistent with MtrC, MtrA, and MtrB forming a complex.
FIG. 4.
(A) Western blots of MtrC/A/B proteins separated by native PAGE. Fractions 38 to 56 shown in Fig. 2 containing the peak MtrC/A/B fractions were pooled, and 15-μl samples were applied to the gel. OM proteins MtrC, MtrB, and MtrA were probed with polyclonal antibodies. (B) TM proteins (15 μg per lane) separated using native PAGE and probed for OmcA and MtrC. Protein bands are indicated with arrows at the sides of the gels.
To demonstrate the specificity of the interaction between MtrC, -A, and -B on the native gel, we ran a similar gel with TM (7.5 μg protein). The TM, in addition to MtrC/A/B, contained OmcA. Probing adjacent lanes with anti-OmcA and anti-MtrC showed that these two OM proteins did not comigrate on the native gel.
Molecular mass determination of the MtrC/A/B complex.
To assess the MtrC/A/B solution molecular mass in 5% (wt/vol) Triton X-100, analytical ultracentrifugation (sedimentation equilibrium) analysis was employed. Sedimentation equilibrium profiles of MtrC/A/B samples were collected at three rotor speeds (6,000, 8,000, and 10,000 rpm) for two MtrC/A/B concentrations (2.5 and 0.25 μM). At each MtrC/A/B concentration, the sedimentation equilibrium data collected at the three rotor speeds were simultaneously fitted to a single-species, noninteracting model and yielded molecular masses of 198 and 197 kDa for the 2.5 and 0.25 μM MtrC/A/B samples, respectively. A representative plot displaying sedimentation behavior of the 2.5 μM MtrC/A/B sample at the three rotor speeds is displayed in Fig. 5A.
FIG. 5.
(A) Analytical ultracentrifugation (sedimentation equilibrium) analysis of MtrC/A/B. A representative plot of absorbance profiles (measured at an absorbance value of 530 nm) of 2.5 μM MtrC/A/B following centrifugation runs at 6,000 (squares), 8,000 (triangles), and 10,000 rpm (diamonds) (16 h at 20°C). The data from all three centrifugation speeds were simultaneously fitted to the equation for a single-species, noninteracting model (solid lines). Conditions of measurement were MtrC/A/B samples in 50 mM HEPES, 200 mM NaCl, 5% (wt/vol) Triton X-100, pH 7.6. Residuals between the experimental data and the fitted lines are shown in the graph at the top of the figure. (B) Representative visualization of sedimentation equilibrium profiles of two concentrations of MtrC/A/B, 2.5 μM (squares) and 0.25 μM (circles) measured at absorbance values of 530 nm and 440 nm, respectively, following centrifugation at 6000 rpm for 16 h at 20°C. Plots of ln absorbance versus (r2 − r02)/2 of both MtrC/A/B samples display similar straight-line slopes, demonstrating single-species, homogeneous system and a derived molecular mass that is independent of concentration in the range examined. Conditions of measurement were MtrC/A/B samples in 50 mM HEPES, 200 mM NaCl, 5% (wt/vol) Triton X-100, pH 7.6. Residuals between the experimental data and the fitted lines are shown in the graph at the top of the figure.
A plot of ln absorbance versus (r2 − r02)/2 (Fig. 5B) is expected to give a straight line, the slope of which is directly proportional to the solution molecular mass of the complex (plus adsorbed detergent) and is independent of concentration for a homogeneous single-component system (15). A representative straight line plot obtained for MtrC/A/B in 5%(wt/vol) Triton X-100 at 6,000 rpm is displayed (Fig. 5B) and demonstrates almost identical gradients at both MtrC/A/B concentrations. This analysis suggests an unchanging molecular mass over the 10-fold concentration range examined and is suggestive of a homogeneous, single-component sample.
Electron transfer from reduced MtrC/A/B complex to various TEAs.
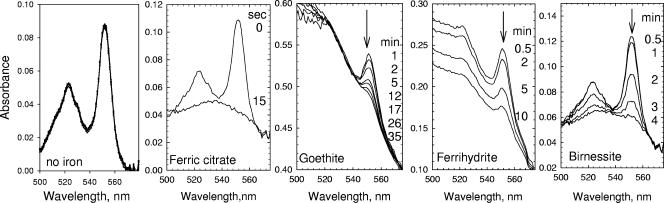
The reactivity of the MtrC/A/B putative complex was demonstrated by spectroscopic studies. MtrC/A/B was purified by anion-exchange chromatography and then gel filtration (Fig. 3). Incremental amounts of dithionite were added to MtrC/A/B until the complex was fully reduced as monitored by absorbance at 550 nm. This fully reduced MtrC/A/B was stable for over 40 min with no autoxidation in the glovebox (Fig. 6). However, it was rapidly oxidized by aqueous ferric citrate and by the solid-phase six-line ferrihydrite, goethite, and birnessite (Fig. 6). While the ferric citrate resulted in oxidation of the complex in less than 15 s, complete oxidation of the complex was much slower with the solid phases: birnessite (4 min), six-line ferrihydrite (10 min), and goethite (35 min).
FIG. 6.
Reduction of various iron and manganese forms with chemically reduced MtrC/A/B. MtrC/A/B from the Sephacryl S-300 column was reduced by dithionite. To the reduced complex, we added either no iron (control), ferric citrate (1 mM), goethite (1 mg/ml), ferrihydrite (2 mM), or birnessite (1 mg/ml). The no-iron addition was scanned at 0, 10, 20, 30, and 40 min, whereas the other samples were scanned at the time intervals shown in the figures. The arrow indicates direction of spectral change going from fully reduced to fully oxidized.
Localization of MtrA.
Our work suggests that MtrA, while weakly associated with MtrB/C is associated with the OM through these protein-protein interactions. Beliaev et al. (5) have shown more of a periplasm localization. To investigate this possibility, TM, OM, CM, and the periplasm were characterized. The TM, OM, and CM fractions were isolated from ferric citrate-grown cultures of S. oneidensis MR-1. Marker enzyme assays were used to determine the degree of separation between the OM and CM. The ratio of NADH oxidase (marker enzyme for the CM) activities in the CM to OM was 0.38 μmol/min·mg protein to 0.05 μmol/min·mg protein. This ratio of 7.6 is comparable to previous results (48), showing that the membrane fractions were well separated. The TM, OM, and CM fractions were then subjected to SDS-PAGE and transferred to nitrocellulose membranes for Western blot analysis. Figure 7A shows that MtrA and MtrB were both found in the TM fraction and the OM fraction. In contrast, the CM protein, CymA, was found in the CM and TM fractions.
FIG. 7.
(A) Separation of proteins from S. oneidensis MR-1 cultures into the CM, OM, and TM fractions. MtrA is shown to fractionate with the OM as does MtrB. CymA associates with the cytosolic membrane. (B) Heme staining of S. oneidensis cell extracts (lane 1), supernatant of washed cells (lane 2), and periplasmic fraction (lane 3). (C) Western blot using anti-MtrA of the same fractions as in panel B. The positions of molecular mass markers (in kilodaltons) are indicated to the left of the gel in panels B and C.
To isolate the periplasmic fraction, cells were osmotically shocked and then centrifuged. The supernatant contained the periplasmic fraction and the pellet contained the whole spheroplasts. Heme staining of Western blots from SDS-PAGE of the periplasmic fraction showed no heme bands with a molecular mass equal to that of MtrA (Fig. 7B). The periplasm did contain heme bands with molecular masses of 10 kDa and 60 kDa which are consistent with previous results (59). In contrast, heme staining of lanes containing whole cells or spheroplasts (prior to osmotic shock) showed a 32-kDa heme band (Fig. 7B). Western blot analyses of the same samples indicated that MtrA is found in whole cells and in spheroplasts. These results are consistent with MtrA being associated mainly with the OM through weak protein-protein interactions at the periplasmic face.
Formaldehyde cross-linking.
Protein-protein interactions were further probed by formaldehyde cross-linking. Whole cells were applied to SDS-polyacrylamide gels and visualized by heme staining. The gel showed four prominent bands which correspond to the molecular masses of OmcA, MtrC, MtrA, and CymA (Table 2). Using antibodies specific for each of these proteins, identical bands were detected at the corresponding molecular masses (Fig. 8). Addition of 1% formaldehyde to the whole cells resulted in formation of new heme bands of approximately 160, 151, 136, and 59 kDa (Fig. 8). The intensity of the new cross-linked bands increased with duration of formaldehyde treatment (Fig. 8). The rightmost lane of the gel shown in Fig. 8 (and also Fig. 9) is the final time point boiled to demonstrate the reversibility of the formaldehyde cross-linking.
TABLE 2.
Physical properties of selected proteins
| Protein | Molecular mass (kDa) | pI |
|---|---|---|
| CctAa | 9.4 | 4.6 |
| CymAa | 21 | 8.7 |
| MtrAa | 32 | 7.8 |
| MtrB | 76 | 4.4 |
| MtrCa | 75 | 5.5 |
| OmcAa | 83 | 6.2 |
| FdnH | 33 | 5.2 |
Heme protein.
FIG. 8.
Heme staining of cells cross-linked by 1% formaldehyde and transferred to a nitrocellulose membrane. Lane 1, untreated cells that were boiled; lane 2, cells treated for 0 min; lane 3, cells treated for 30 min; lane 4, cells treated for 60 min; lane 5, cells treated for 120 min; lane 6, cells treated for 120 min and boiled. Each lane was loaded with 1 absorbance unit of cells to increase the concentration of low-abundance heme proteins. The arrows on the left indicate the bands that are observed, and the heme proteins OmcA, MtrC, MtrA, and CymA are identified. The new heme protein bands as a result of treatment with formaldehyde are indicated by arrows without labels. The positions of molecular mass markers (in kilodaltons) are indicated to the right of the membrane.
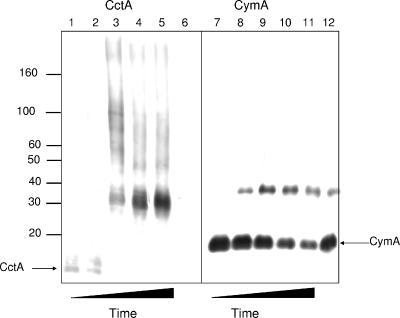
FIG. 9.
In vivo cross-linking of cells grown with ferric citrate as the terminal electron acceptor probed for complexes containing OmcA (A), MtrC (B), MtrB (C), MtrA (D), and CymA (E). Lanes 1, untreated cells that were boiled; lanes 2 to 5, cells treated with 1% formaldehyde for 0 min (lanes 2), 30 min (lanes 3), 60 min (lanes 4), and 120 min (lanes 5); lanes 6, cells treated with 1% formaldehyde for 120 min and boiled. The positions of formaldehyde cross-linked complexes are indicated by arrows to the left of the gels, and the molecular mass markers are indicated to the right of the gels.
Antibodies were used to identify the cross-linked proteins. OmcA cross-reactive material was detected in bands at 83 kDa (non-cross-linked OmcA) and approximately 130 and 160 kDa (Fig. 9). Two other bands were detected at molecular masses higher than 220 kDa, beyond the resolution range of our gels. MtrC cross-reactive material was detected at the 75-kDa band (non-cross-linked) and at the 160-kDa band. This 160-kDa band is identical in size to the OmcA cross-reactive band and thus is consistent with a one-to-one complex of OmcA and MtrC (calculated molecular mass of 158 kDa).
MtrB cross-reactive material was detected at 76 kDa (native non-cross-linked), 151 and 136 kDa (Fig. 9). The cross-linking was extensive with MtrB. The monomer proteins disappeared during the time course of the experiment. Western blots using MtrA antibody show the same higher-molecular-mass complexes detected with anti-MtrB: approximately 136 and 151 kDa (Fig. 9). The 136-kDa band, although cross-reactive with both MtrA and MtrB, was larger than a one-to-one complex (which would be 108 kDa). Thus, proteins of approximately 28 kDa and 15 kDa appear to be found in the complex. These two bands (136 and 151 kDa) were also detected in the heme-stained cross-link gel blot (Fig. 8). The monomeric form of MtrA was completely cross-linked during the time course of the experiment.
Cross-linking of CM and periplasmic proteins CymA and FDH was also examined. CymA cross-reactive material was detected at 19 (non-cross-linked CymA), 32, 54, 95, and 116 kDa (Fig. 9). With FDH, we used antibodies raised to the H-subunit of the respiratory formate dehydrogenase (FdnH). While we were able to detect cross-linking of the H-subunit with what would be the G- and I-subunits by molecular mass, no other cross-reactive bands could be detected (data not shown). Since these proteins are thought to exchange electrons via the menaquinone pool rather than direct protein-protein interactions, this result is expected and gives confidence in the specificity of the interactions detected by our cross-linking experiments.
Periplasm-localized CctA has a molecular mass of 12.1 kDa. If CctA is cross-linked to CymA, it would yield a complex with a molecular mass of approximately 30 kDa, which is close to the 32-kDa band observed with the CymA antibody (Fig. 9). Using a higher percentage SDS-polyacrylamide gel, we examined more closely the possible cross-linking between CctA and CymA. The results clearly show that although the molecular masses of the two cross-reactive bands are close, they are slightly different. The results with the CctA antibody also did not reveal interactions between CctA and MtrA (Fig. 10).
FIG. 10.
In vivo cross-linking of cells grown with ferric citrate as the terminal electron acceptor probed for complexes containing CctA and CymA. Lanes 1 and 7, untreated cells and boiled; lanes 2 and 8, cells treated with 1% formaldehyde for 0 min; lanes 3 and 9, cells treated with 1% formaldehyde for 30 min; lanes 4 and 10, cells treated with 1% formaldehyde for 60 min; lanes 5 and 11, cells treated with 1% formaldehyde for 120 min; lanes 6 and 12, cells treated with 1% formaldehyde for 120 min and boiled. The positions of formaldehyde cross-linked complexes are indicated by arrows at the sides of the gels, and the positions of molecular mass markers (in kilodaltons) are indicated to the left of the gel.
DISCUSSION
In the present study, we have characterized protein-protein interactions of the OM, periplasm, and CM of S. oneidensis using protein purification and cross-linking methods. Our results show the following for the first time. (i) MtrC, MtrA, and MtrB exist as a complex in the OM. (ii) The purified MtrC/A/B complex has a molecular mass of 198 kDa, consistent with a 1:1:1 stoichiometry of the MtrC, MtrA, and MtrB subunits. (iii) MtrC/A/B has aqueous Fe and Fe and Mn solid-oxide reduction capabilities. (iv) MtrA is associated with the OM, presumably at the periplasmic face but is not freely diffusible in the periplasm. (v) MtrC weakly interacts with OmcA and does not form a complex that is stable during purification using the methodologies that yield a stable MtrC/A/B complex. While our negative results do not negate such interactions, we have not been able to demonstrate (i) interaction between CctA and CymA and (ii) interaction between CctA and MtrA. The implications of these findings are discussed below.
Electron transport through the CM.
A model of the protein-protein interactions consistent with these new and previously published results is shown in Fig. 11. Proton motive force-generating respiratory electron transfer in the CM is initiated by oxidation of periplasmic formate to CO2 by FDH (51). Reduced FDH then reduces menaquinone by two electrons to menaquinol (translocating 2 H+ to the periplasm), which in turn transfers its electrons to CymA (46). Our cross-linking experiments did not reveal any protein partners for FDH or CymA, which is consistent with these proteins interacting with the MQ/MQH2 pool rather than directly with each other. CymA, though, plays a central role in respiration, since knockouts of CymA are defective in utilization of all TEAs except for trimethylamine N-oxide by Shewanella (30, 34).
FIG. 11.
Model depicting the interactions of OM proteins as a result of cross-linking and native protein methods. Interactions of CymA and CctA are yet to be defined. Menaquinone is shown in the membrane and shown as “Q” in the figure. The interaction of MtrC/A/B as a complex has been demonstrated in this work. We and others (52) have demonstrated the interaction of OmcA and MtrC. MtrA faces the periplasm, while OmcA and MtrC are extracellularly exposed lipoproteins and may potentially contact mineral oxides. The reduction potentials of various proteins and electron acceptors are shown to the left. Reduction potentials were obtained from the following: formate (62), menaquinone (42), MtrA (44), CctA (11), EDTA-Fe3+ (49), NTA-Fe3+, goethite, and citrate-Fe3+ (58), and pyrolusite (55). Decoupling of the electron transfer to insoluble TEAs from the proton pump at the CM implies that electron transfer to solid oxides does not provide energy to the cell but serves only to ground the cell by dumping electrons.
Electron transport through the periplasm.
For soluble TEAs, electron transfer has been proposed to occur at the CM in the periplasm (28, 35), which is consistent with our in vitro iron reduction studies (47). For solid-phase TEAs, electrons of CM-localized CymA must traverse the periplasm and through the OM. Electron transfer through the periplasm occurs either by continuous contact of proteins spanning the periplasm or by shuttling electron carriers in the periplasmic. Two candidate proteins that can span the periplasm or can act as shuttles are MtrA and CctA. Our cross-linking experiments with the CymA antibody, while not excluding the possibility, were not able to show any interaction between these two proteins. In contrast, high-molecular-mass complexes are observed with the CymA antibody (34, 54, 95, and 116 kDa) that might be involved with electron shuttling in the periplasm; however, these complexes could not be identified.
The apparent lower affinity of MtrA for the MtrC/A/B complex could confer upon this protein the ability to act as a reversible periplasmic electron shuttle (i.e., MtrA reversibly associates/dissociates with MtrB/C depending on the redox state of the protein). However, if MtrA served as a shuttle between the OM and CM, one would predict major localization as a freely diffusible protein in the periplasm, and this was not observed. Our results therefore argue against, but do not definitively preclude, MtrA being a diffusible periplasmic electron shuttle. The periplasm has an estimated inner membrane-to-CM width of around 150Å. Recent structures of the 20-kDa pentaheme NrfB protein reveal an electron wire of around 40Å (7). Thus, the 40-kDa decaheme MtrA could conceivably have a heme wire of some 80 Å allowing it to penetrate some distance from the OM into periplasm, but possibly insufficient to directly contact CymA at the inner membrane. Our observations are then at present best interpreted as revealing that MtrA serves as the protein that receives electrons at the OM.
Another possible periplasmic electron shuttle is CctA which is periplasm localized based on signal sequence predictions (5). Our cross-linking experiments were not able to identify protein-protein partners with CctA. Its molecular mass (10 kDa) is approximately consistent with the difference between the 151- and 136-kDa bands which are known to contain MtrA and MtrB. However, further characterization with a CctA antibody revealed only a 28-kDa cross-reactive band. In regard to its interaction with CM proteins, the 28-kDa protein also does not correspond with a complex which would be formed with CymA. Thus, our experiments were not able to identify the electron transfer chain through CctA.
Electron transport through the OM.
In contrast to the periplasm, our results provide a well-defined model for the OM (Fig. 11). In terms of OM proteins, OmcA could be easily purified; in contrast, MtrC was not only difficult to purify, it was also unstable with decreasing yields during the purification process. This is consistent with an obligate association with other protein-forming complexes. Anion-exchange chromatography suggests that these other subunits are MtrA and MtrB. These proteins coeluted with MtrC despite pI values of 7.78 (MtrA), 4.42 (MtrC), and 5.48 (MtrB). With a pI of 6.23 for OmcA, the elution order from an anion-exchange column should be MtrA, OmcA, MtrC, and then MtrB (first to last). In contrast, OmcA consistently eluted first, before MtrA. Furthermore, despite a pI difference of 1.06, MtrC and MtrB coeluted as one peak, consistent with a strong complex between the two proteins. Although some MtrA eluted before MtrB and MtrC, most eluted together with those proteins, consistent with a three-subunit OM complex.
The existence of MtrC/A/B as a complex is also consistent with native gel electrophoresis. Separating based on size and charge, MtrC, MtrA, and MtrB migrated as (at least) two common bands. The presence of additional bands indicates the presence of more than one form of the protein in addition to potential monomers. Finally, an MtrC/A/B complex is consistent with the fact that these three proteins are expressed as part of an operon (5).
The observed molecular mass of the MtrC/A/B complex (197 to 198 kDa) is close to that predicted for a 1:1:1 MtrC:MtrA:MtrB stoichiometry based on primary sequence analysis and sedimentation equilibrium analysis of purified MtrC (∼85 kDa) and MtrA (∼40 kDa) (data not shown) determined in identical buffer/detergent conditions. MtrB has not yet been characterized by analytical ultracentifugation, as pure protein is not available, but from primary sequence analysis, a molecular mass of 75 kDa is predicted. Thus, the total predicted mass of a 1:1:1 complex of MtrC/A/B is ∼200 kDa, which is very close to the experimentally derived figure. When purified, this complex has the ability to reduce multiple metal forms, including aqueous and solid species (ferric citrate, six-line ferrihydrite, goethite, and birnessite). The reactivity of the metal forms vary where the amount of time needed to fully oxidize the MtrC/A/B complex span from <15 seconds for the aqueous species to up to 35 min for solid phases.
Formaldehyde protein cross-linking experiments indicate little, if any, cross-linking between MtrB and MtrC, though these two proteins exhibited the highest degree of association in chromatographic and electrophoretic separation. The lack of cross-linking between MtrB and MtrC does not negate interactions, since the cross-linking reaction is dependent upon basic side chain residues in close enough proximity for formaldehyde cross-linking to occur (45). In contrast, although MtrA and MtrB are extensively cross-linked, in the chromatography experiments, MtrA exhibited a lower binding affinity, as it was partially separated from MtrB and MtrC. Even though the binding affinity of MtrA is lowest with the putative MtrC/A/B complex, it is the most efficiently cross-linked, which is consistent with MtrA and MtrB having reactive amines in close proximity.
Our results also show that MtrC/A/B interacts with OmcA through MtrC, in agreement with the findings of Shi et al. (52). These workers reported the interaction between OmcA and MtrC as a high-affinity complex characterized by a dissociation constant of less than 0.5 μM. While our results support an interaction between these two proteins, it is under our experimental conditions a less stable interaction compared to that of MtrC with MtrA and with MtrB. For example, our results show that the interaction between OmcA and MtrC is disrupted by anion-exchange chromatography, whereas the MtrC/A/B interaction is not. Also, native gel electrophoresis suggests that the interaction between these two proteins is low in affinity and transient in nature. Thus, the stability of the interaction between OmcA and MtrC may be dependent on growth and/or experimental conditions, and this requires further study.
Electron transfer to solid-phase oxides.
In the absence of soluble TEAs, electrons are transported across the periplasm to the OM, where their interactions with the TEAs most likely occur through OmcA and MtrC. Based on current knowledge, OM-localized electrons cannot participate in proton motive force-generating electron transfer (Fig. 11). Thus, these OM electron carriers serve only to ground the cell by transferring electrons out. To act as effective grounding wires, the electron carriers across the OM would exhibit very low substrate specificity. Such may be the case, since Shewanella can apparently reduce both solid Mn and Fe oxides with the same suite of enzymes. Furthermore, global transcriptome analysis shows no significant differences in gene expression by S. oneidensis when grown on solid ferrihydrite, MnO2, or colloidal Mn (3). In addition, no mutants that can use Mn solid oxide as a TEA but not Fe solid oxide have been isolated (T. J. DiChristina, personal communication). Efficient grounding for the cell may also occur if both of the OM heme proteins provide separate routes of electron transfer (19).
Importantly, if the reduction of insoluble TEAs at the OM is decoupled from proton pumping at the CM, then microbes cannot exploit the greater reduction potential of solid-phase TEAs for energy generation. Consistent with this, Kostka et al. (17) found no difference in cell yield (measured as carbon yield) for S. oneidensis grown on soluble ferric citrate versus insoluble iron oxides when calculated per mole of electrons utilized. Furthermore, based upon graphed data reported by DiChristina et al. (8), approximately identical yields (∼4.1 versus 4.9 × 1013 cells/mol of electrons) were observed when S. oneidensis was grown on ferric citrate versus Mn oxide, respectively.
Despite the observations of apparently equal growth yields for aqueous versus solid-phase TEAs in the laboratory, in nature, Mn oxides are generally reduced earlier (e.g., at shallower depths) than Fe oxides (57). This has been explained by arguing that organisms that utilize TEAs with higher reduction potential outcompete organisms utilizing TEAs lower on the redox ladder due to higher growth yields. However, based upon Fig. 11, utilization of Mn before Fe oxides must only reflect that solid-phase metal oxides with higher reduction potential are used up first simply due to the faster kinetics of reduction of these phases. To maximize cell yield, microorganisms can regulate the use of soluble TEAs through gene expression, but cell yield is not affected by gene regulation of the use of solid-phase TEAs. Transfer of electrons occurs faster into metal oxides with higher reduction potential due to kinetics (kinetic control) but not through gene-enabled substrate-specific binding between the OM proteins and the insoluble oxides (regulatory control).
Acknowledgments
This research was supported by DOE grant DE-FG02-01ER15209, the NASA Astrobiology Institute (NASA Astrobiology Institute Cooperative Agreement NCC2-1057), the NSF Environmental Molecular Science Institute program (CHE-0431328) (for the Center for Environmental Kinetics Analysis), and the Penn State Biogeochemical Research Initiative for Education (BRIE), sponsored by NSF (IGERT) grant DGE-9972759. D. J. Richardson thanks the BBSRC for support through grants B18695 and BBSSA200410938 and the DOE for support through a Biogeochemical Grand Challenge.
We thank Kathleen Postle for advice on all of the protein cross-linking studies. Special thanks go to Lucien Weiss for purification of recombinant heme proteins and antibody preparation and to Hasan Koc for performing liquid chromatography-tandem mass spectrometry identification of proteins.
Footnotes
Published ahead of print on 3 August 2007.
REFERENCES
- 1.Arnold, R. G., T. J. DiChristina, and M. R. Hoffmann. 1986. Inhibitor studies of dissimilative Fe(III) reduction by Pseudomonas sp. strain 200 (“Pseudomonas ferrireductans”). Appl. Environ. Microbiol. 52:281-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arslan, E., H. Schulz, R. Zufferey, P. Kunzler, and L. Thony-Meyer. 1998. Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 251:744-747. [DOI] [PubMed] [Google Scholar]
- 3.Beliaev, A. S., D. M. Klingeman, J. A. Klappenbach, L. Wu, M. F. Romine, J. M. Tiedje, K. H. Nealson, J. K. Fredrickson, and J. Zhou. 2005. Global transcriptome analysis of Shewanella oneidensis MR-1 exposed to different terminal electron acceptors. J. Bacteriol. 187:7138-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beliaev, A. S., and D. A. Saffarini. 1998. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J. Bacteriol. 180:6292-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beliaev, A. S., D. A. Saffarini, J. L. McLaughlin, and D. Hunnicutt. 2001. MtrC, an outer membrane decahaem c cytochrome required for metal reduction in Shewanella putrefaciens MR-1. Mol. Microbiol. 39:722-730. [DOI] [PubMed] [Google Scholar]
- 6.Brunauer, S. 1987. About some critics of the BET theory. Langmuir 3:3-4. [Google Scholar]
- 7.Clarke, T., J. Cole, D. Richardson, and A. Hemmings. 2007. The crystal structure of the pentahaem c-type cytochrome NrfB and characterisation of its solution-state interaction with the pentahaem nitrite reductase NrfA. Biochem. J. 406:19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiChristina, T. J., C. M. Moore, and C. A. Haller. 2002. Dissimilatory Fe(III) and Mn(IV) reduction by Shewanella putrefaciens requires ferE, a homolog of the pulE (gspE) type II protein secretion gene. J. Bacteriol. 184:142-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredrickson, J. K., H. M. Kostandarithes, S. W. Li, A. E. Plymale, and M. J. Daly. 2000. Reduction of Fe(III), Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1. Appl. Environ. Microbiol. 66:2006-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredrickson, J. K., J. M. Zachara, D. W. Kennedy, H. L. Dong, T. C. Onstott, N. W. Hinman, and S. M. Li. 1998. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta 62:3239-3257. [Google Scholar]
- 11.Gordon, E. H. J., A. D. Pike, A. E. Hill, P. M. Cuthbertson, S. K. Chapman, and G. A. Reid. 2000. Identification and characterization of a novel cytochrome c(3) from Shewanella frigidimarina that is involved in Fe(III) respiration. Biochem. J. 349:153-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyodo, Y., S. K. Banerjee, R. L. Penn, D. Burleson, T. S. Berauo, T. Seda, and P. Solheid. 2006. Magnetic properties of synthetic six-line ferrihydrite nanoparticles. Phys. Earth Planet. Interact. 154:222-233. [Google Scholar]
- 13.Herbel, M. J., J. S. Blum, R. S. Oremland, and S. E. Borglin. 2003. Reduction of elemental selenium to selenide: experiments with anoxic sediments and bacteria that respire Se-oxyanions. Geomicrobiol. J. 20:587-602. [Google Scholar]
- 14.Higgs, P. I., P. S. Myers, and K. Postle. 1998. Interactions in the TonB-dependent energy transduction complex: ExbB and ExbD form homomultimers. J. Bacteriol. 180:6031-6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horan, T., J. Wen, T. Arakawa, N. L. Liu, D. Brankow, S. Hu, B. Ratzkin, and J. S. Philo. 1995. Binding of Neu differentiation factor with the extracellular domain of Her2 and Her3. J. Biol. Chem. 270:24604-24608. [DOI] [PubMed] [Google Scholar]
- 16.Kaderbhai, M. A., D. J. Hopper, K. M. Akhtar, S. K. Abbas, and N. N. Kaderbhai. 2003. A cytochrome c from a lupanine-transforming Pseudomonas putida strain is expressed in Escherichia coli during aerobic cultivation and efficiently exported and assembled in the periplasm. Appl. Environ. Microbiol. 69:4727-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kostka, J. E., G. W. Luther, and K. H. Nealson. 1995. Chemical and biological reduction of Mn(III)-pyrophosphate complexes—potential importance of dissolved Mn(III) as an environmental oxidant. Geochim. Cosmochim. Acta 59:885-894. [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Lies, D. P., M. E. Hernandez, A. Kappler, R. E. Mielke, J. A. Gralnick, and D. K. Newman. 2005. Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms. Appl. Environ. Microbiol. 71:4414-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, C., J. M. Zachara, Y. A. Gorby, J. E. Szecsody, and C. F. Brown. 2001. Microbial reduction of Fe(III) and sorption/precipitation of Fe(II) on Shewanella putrefaciens strain CN32. Environ. Sci. Technol. 35:1385-1393. [DOI] [PubMed] [Google Scholar]
- 21.Lopano, C. L., P. J. Heaney, J. E. Post, J. Hanson, and S. Komarneni. 2007. Time-resolved structural analysis of K- and Ba-exchange reactions with synthetic Na-birnessite using synchrotron X-ray diffraction. Am. Miner. 92:380-387. [Google Scholar]
- 22.Lovley, D. R. 2002. Dissimilatory metal reduction: from early life to bioremediation. ASM News 68:231-237. [Google Scholar]
- 23.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 25.Madigan, M. T., J. M. Martinko, and J. Parker. 2000. Brock biology of microorganisms. Prentice Hall, Upper Saddle River, NJ.
- 26.Marchese-Ragona, S. P., J. S. Wall, and K. A. Johnson. 1988. Structure and mass analysis of 14S dynein obtained from Tetrahymena cilia. J. Cell Biol. 106:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moser, D. P., and K. H. Nealson. 1996. Growth of the facultative anaerobe Shewanella putrefaciens by elemental sulfur reduction. Appl. Environ. Microbiol. 62:2100-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers, C. R., B. P. Carstens, W. E. Antholine, and J. M. Myers. 2000. Chromium(VI) reductase activity is associated with the cytoplasmic membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Appl. Microbiol. 88:98-106. [DOI] [PubMed] [Google Scholar]
- 29.Myers, C. R., and J. M. Myers. 2003. Cell surface exposure of the outer membrane cytochromes of Shewanella oneidensis MR-1. Lett. Appl. Microbiol. 37:254-258. [DOI] [PubMed] [Google Scholar]
- 30.Myers, C. R., and J. M. Myers. 1997. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens MR-1. J. Bacteriol. 179:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers, C. R., and J. M. Myers. 1994. Ferric iron reduction-linked growth yields of Shewanella putrefaciens MR-1. J. Appl. Bacteriol. 76:253-258. [DOI] [PubMed] [Google Scholar]
- 32.Myers, C. R., and J. M. Myers. 1992. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Bacteriol. 174:3429-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers, C. R., and J. M. Myers. 2002. MtrB is required for proper incorporation of the cytochromes OmcA and OmcB into the outer membrane of Shewanella putrefaciens MR-1. Appl. Environ. Microbiol. 68:5585-9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers, C. R., and J. M. Myers. 1993. Role of menaquinone in the reduction of fumarate, nitrate, iron(III) and manganese(IV) by Shewanella putrefaciens MR-1. FEMS Microbiol. Lett. 114:215-222. [Google Scholar]
- 35.Myers, C. R., and J. M. Myers. 2004. Shewanella oneidensis MR-1 restores menaquinone synthesis to a menaquinone-negative mutant. Appl. Environ. Microbiol. 70:5515-5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 37.Myers, C. R., and K. H. Nealson. 1988. Microbial reduction of manganese oxides: interactions with iron and sulfur. Geochim. Cosmochim. Acta 52:2727-2732. [Google Scholar]
- 38.Myers, J. M., and C. R. Myers. 2001. Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide. Appl. Environ. Microbiol. 67:260-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nealson, K. H., A. Belz, and B. McKee. 2002. Breathing metals as a way of life: geobiology in action. Antonie Leeuwenhoek 81:215-222. [DOI] [PubMed] [Google Scholar]
- 40.Newman, D. K., T. J. Beveridge, and F. M. M. Morel. 1997. Precipitation of arsenic trisulfide by Desulfotomaculum auripigmentum. Appl. Environ. Microbiol. 63:2022-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osborn, M. J., J. E. Gander, E. Parisi, and J. Carson. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J. Biol. Chem. 247:3962-3972. [PubMed] [Google Scholar]
- 42.Patel, K. B., and R. L. Willson. 1973. Semiquinone free radicals and oxygen. Pulse radiolysis study of one electron transfer equilibra. J. Chem. Soc. Faraday Trans. 69:814-825. [Google Scholar]
- 43.Philo, J. S. 1997. An improved function for fitting sedimentation velocity data for low-molecular-weight solutes. Biophys. J. 72:435-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pitts, K. E., P. S. Dobbin, F. Reyes-Ramirez, A. J. Thomson, D. J. Richardson, and H. E. Seward. 2003. Characterization of the Shewanella oneidensis MR-1 decaheme cytochrome MtrA. J. Biol. Chem. 278:27758-27765. [DOI] [PubMed] [Google Scholar]
- 45.Prossnitz, E., K. Nikaido, S. J. Ulbrich, and G. F. Ames. 1988. Formaldehyde and photoactivatable cross-linking of the periplasmic binding protein to a membrane component of the histidine transport system of Salmonella typhimurium. J. Biol. Chem. 263:17917-17920. [PubMed] [Google Scholar]
- 46.Richardson, D., and G. Sawers. 2002. PMF through the redox loop. Science 295:1842-1843. [DOI] [PubMed] [Google Scholar]
- 47.Ruebush, S. S., S. L. Brantley, and M. Tien. 2006. Reduction of soluble and insoluble iron forms by membrane fractions of Shewanella oneidensis grown under aerobic and anaerobic conditions. Appl. Environ. Microbiol. 72:2925-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruebush, S. S., G. A. Icopini, S. L. Brantley, and M. Tien. 2006. In vitro enzymatic reduction kinetics of mineral oxides by membrane fractions from Shewanella oneidensis MR-1. Geochim. Cosmochim. Acta 70:56-70. [Google Scholar]
- 49.Schwarzenbach, G., and J. Heller. 1951. Komplexone XVIII. Die Eisen(II)- und Eisen(III)-komplexe der Athylendiamin-tetraessig-saure und ihr Redoxgleichgewicht. Helv. Chim. Acta 34:576-591. [Google Scholar]
- 50.Schwertmann, U., and R. M. Cornell. 1991. Iron oxides in the laboratory: preparation and characterization, p. 137. In R. M. Cornell and U. Schwertmann (ed.), The iron oxides: structure, properties, reactions, occurrences and uses. John Wiley & Sons, New York, NY.
- 51.Scott, J. H., and K. H. Nealson. 1994. A biochemical study of the intermediary carbon metabolism of Shewanella putrefaciens. J. Bacteriol. 176:3408-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi, L., B. Chen, Z. Wang, D. A. Elias, M. U. Mayer, Y. A. Gorby, S. Ni, B. H. Lower, D. W. Kennedy, D. S. Wunschel, H. M. Mottaz, M. J. Marshall, E. A. Hill, A. S. Beliaev, J. M. Zachara, J. K. Fredrickson, and T. C. Squier. 2006. Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c-type cytochromes of Shewanella oneidensis MR-1. J. Bacteriol. 188:4705-4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shyu, J. B. H., D. P. Lies, and D. K. Newman. 2002. Protective role of tolC in efflux of the electron shuttle anthraquinone-2,6-disulfonate. J. Bacteriol. 184:1806-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinle, A., P. Li, D. L. Morris, V. Groh, L. L. Lanier, R. K. Strong, and T. Spies. 2001. Interactions of human NKG2D with its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics 53:279-287. [DOI] [PubMed] [Google Scholar]
- 55.Stone, A. T., and H. J. Ulrich. 1989. Kinetics and reaction stoichiometry in the reductive dissolution of manganese(IV) dioxide and Co(III) oxide by hydroquinone. J. Colloid Interface Sci. 132:509-522. [Google Scholar]
- 56.Stookey, L. L. 1970. Ferrozine: a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 57.Stumm, W., and J. J. Morgan. 1996. Aquatic chemistry. John Wiley & Sons, New York, NY.
- 58.Thamdrup, B. 2000. Bacterial manganese and iron reduction in aquatic sediments. Adv. Microb. Ecol. 16:1-84. [Google Scholar]
- 59.Tsapin, A. I., I. Vandenberghe, K. H. Nealson, J. H. Scott, T. E. Meyer, M. A. Cusanovich, E. Harada, T. Kaizu, H. Akutsu, D. Leys, and J. J. Van Beeumen. 2001. Identification of a small tetraheme cytochrome c and a flavocytochrome c as two of the principal soluble cytochromes c in Shewanella oneidensis strain MR1. Appl. Environ. Microbiol. 67:3236-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venkateswaran, K., M. E. Dollhopf, R. Aller, E. Stackebrandt, and K. H. Nealson. 1998. Shewanella amazonensis sp. nov., a novel metal-reducing facultative anaerobe from Amazonian shelf muds. Int. J. Syst. Bacteriol. 48:965-972. [DOI] [PubMed] [Google Scholar]
- 61.Wade, R., and T. J. DiChristina. 2000. Isolation of U(VI) reduction-deficient mutants of Shewanella putrefaciens. FEMS Microbiol. Lett. 184:143-148. [DOI] [PubMed] [Google Scholar]
- 62.White, D. 2000. The physiology and biochemistry of prokaryotes. Oxford University Press, Oxford, United Kingdom.
- 63.Xiong, Y., L. Shi, B. Chen, M. U. Mayer, B. H. Lower, Y. Londer, S. Bose, M. F. Hochella, J. K. Fredrickson, and T. C. Squier. 2006. High-affinity binding and direct electron transfer to solid metals by the Shewanella oneidensis MR-1 outer membrane c-type cytochrome OmcA. J. Am. Chem. Soc. 128:13978-13979. [DOI] [PubMed] [Google Scholar]