Abstract
The bacterial reduction of actinides has been suggested as a possible remedial strategy for actinide-contaminated environments, and the bacterial reduction of Pu(VI/V) has the potential to produce highly insoluble Pu(IV) solid phases. However, the behavior of plutonium with regard to bacterial reduction is more complex than for other actinides because it is possible for Pu(IV) to be further reduced to Pu(III), which is relatively more soluble than Pu(IV). This work investigates the ability of the metal-reducing bacteria Geobacter metallireducens GS15 and Shewanella oneidensis MR1 to enzymatically reduce freshly precipitated amorphous Pu(IV) (OH)4 [Pu(IV)(OH)4(am)] and soluble Pu(IV)(EDTA). In cell suspensions without added complexing ligands, minor Pu(III) production was observed in cultures containing S. oneidensis, but little or no Pu(III) production was observed in cultures containing G. metallireducens. In the presence of EDTA, most of the Pu(IV)(OH)4(am) present was reduced to Pu(III) and remained soluble in cell suspensions of both S. oneidensis and G. metallireducens. When soluble Pu(IV)(EDTA) was provided as the terminal electron acceptor, cell suspensions of both S. oneidensis and G. metallireducens rapidly reduced Pu(IV)(EDTA) to Pu(III)(EDTA) with nearly complete reduction within 20 to 40 min, depending on the initial concentration. Neither bacterium was able to use Pu(IV) (in any of the forms used) as a terminal electron acceptor to support growth. These results have significant implications for the potential remediation of plutonium and suggest that strongly reducing environments where complexing ligands are present may produce soluble forms of reduced Pu species.
Plutonium has a long half-life (2.4 × 104 years) and is very toxic, and a full understanding of its speciation and interactions with environmental processes is required in order to predict, contain, or remediate contaminated sites. Plutonium is present at variable concentrations in nuclear waste repositories, nuclear testing facilities, and sites contaminated by accidental release (6, 11, 25). Plutonium contamination often occurs with a number of other metals, organic compounds, chelating agents, and/or other cocontaminants that could significantly affect its mobility (11, 12, 25). In addition, some areas contaminated with plutonium present challenges that are difficult to effectively treat with traditional remedial approaches.
Currently, there is little information available concerning plutonium behavior in contaminated soils or subsurface environments as a function of natural processes. Understanding the environmental processes that can potentially affect plutonium speciation and mobility will allow better management of Pu-contaminated sites. Microbial processes are among the many environmental processes that can affect Pu speciation. In particular, metal-reducing bacteria, which use oxidized metals as terminal electron acceptors and produce reduced metal species, can potentially have a significant impact on the speciation of Pu in subsurface environments. The reduction of U(VI) by metal-reducing bacteria has been extensively examined as a remediation technique that could be used to stabilize uranium in situ (1, 18). The long-term stability of the reduced forms of actinides and the mobility of the biogenic colloidal species produced are among the important aspects that need to be more fully understood (16, 29). Plutonium in particular poses additional challenges, because the redox potential for the reduction of amorphous Pu(IV)(OH)4 [Pu(IV)(OH)4(am)] has been estimated to be −0.39 mV (8), which is near the lower limit accessible to bacterial reduction.
The possible reduction of Pu(IV) is of concern because Pu(IV) hydrous oxides, which represent the main fraction of Pu contaminants in the environment (8, 9, 17), could be mobilized by biological reduction to the more soluble Pu(III) oxidation state. A single previous study attempted to characterize the reduction of solid PuO2 and subsequent solubilization of Pu(III) by Bacillus polymyxa and Bacillus circulans (28). During this experiment, the solubilization of PuO2 increased by ∼40% in the presence of metal-reducing bacteria. The solubilization of PuO2 was increased to approximately 90% when nitrilotriacetic acid (NTA) was added (28). Although no Pu(III) was observed, the enhanced solubility of Pu was attributed to Pu(IV) reduction to Pu(III), with the reoxidation of Pu(III) to Pu(IV) and the complexation of Pu(IV) with NTA. The study clearly demonstrates that the combination of metabolically active bacteria with complexants can dramatically shift the speciation and stability of plutonium hydrous oxides. However, since only Pu(IV) was observed, the enzymatic reduction of Pu(IV) can only be inferred from this study.
The complexation with organic ligands can affect both the solubility and redox chemistry of Pu. Biogenic chelators, such as siderophores, small organic molecules produced by various microorganisms and plants to acquire iron, and synthetic ligands, such as EDTA, DTPA (diethylenetriamine pentaacetic acid), and NTA used in plutonium processing, can increase Pu species solubility and mobility (27). The complexation of Pu with these organic compounds tends to reduce the reduction potential of Pu, and in general, the stronger the Pu-ligand bond, the lower the redox potential (2). Both the enhanced solubility and the reduced reduction potential can potentially affect the behavior of Pu in soil and subsurface environments.
In this study, we present data on the interactions of Geobacter metallireducens and Shewanella oneidensis with freshly precipitated Pu(OH)4(am) and soluble Pu(IV)(EDTA) under cell suspensions and growth conditions.
MATERIALS AND METHODS
Reagents.
All solutions were prepared with deionized, distilled water. Plutonium(IV) stock solutions were prepared as described elsewhere (24). Briefly, plutonium metal was dissolved in chilled 6 M HCl to give a Pu(III) solution, which was mixed with an equal volume of 16 M HNO3 to give a Pu(IV) solution. The Pu(IV) solution was purified using anion-exchange chromatography using Bayer Lewatit MP-500 macroporous resin. Pu(IV) in 8 M HNO3 was loaded on the resin, washed with 9 M HCl, and then eluted with 0.5 M HCl. Fresh plutonium hydroxide, Pu(OH)4(am), was prepared by hydrolysis of a pure Pu(IV) stock solution with the addition of sodium hydroxide. The green precipitate obtained was washed with distilled water until the pH was neutral and resuspended in the same volume of distilled water as the initial Pu(IV) solution prior to the addition of NaOH. Since the Pu(OH)4(am) was resuspended in the same volume of solution as the initial Pu(IV) solution, an equivalent aqueous concentration was assigned to the Pu(OH)4(am) solution. Stock solutions of Pu were not sterilized other than by the initially low pH of the solution. The suspension of Pu(OH)4(am) was homogenized before each sampling. Pu(IV)(EDTA) was prepared by slowly adding 1 equivalent of acidic Pu(IV) to an aqueous solution containing 1 equivalent of EDTA. The solution was left to equilibrate for 5 min, and then the pH was increased to 7.0. The concentration of the Pu(IV)(EDTA) stock solution was determined spectrophotometrically using the specific absorption band characterizing the complex Pu(IV)(EDTA) (ɛ495 was 48 M−1 cm−1 at pH 2.50). This specific absorption coefficient was determined experimentally by recording the UV-visible spectrum of a 1:1 Pu(IV)-EDTA complex. The concentration of Pu(IV) was determined spectrophotometrically using the specific absorption band at 470 nm characterizing Pu(IV) in strong acid (ɛ470 was 56 M−1 cm−1 in 1.0 M HClO4) (10).
Bacterial strain and culture conditions.
G. metallireducens and S. oneidensis were obtained from the American Type Culture Collection. A detailed description of the growth media used for maintaining cultures and growing cells for experiments can be found elsewhere (15). Briefly, the growth medium composition per liter is 3.4 g NaOH, 12.25 g Fe-citrate (anhydrous), 0.25 g NH4Cl, 1.08 g glycerol-2-PO4, 0.025 g KCl, 10.46 g MOPS (morpholinepropanesulfonic acid), 10.0 ml Wolfe's minerals, 10 ml Thaver's vitamins, and 10 mM acetate (G. metallireducens) or 10 mM lactate (S. oneidensis). Oxygen was removed from the medium by bubbling ultrahigh-purity argon gas through the solution for about 1 h. The solutions were then sealed in serum bottles and autoclaved. The bacteria were grown anaerobically at 37°C in 50-ml cerium bottles or 1.0-liter airtight culture flasks maintained in the dark. Full-grown cultures were transferred anaerobically to centrifuge tubes, and the cells were pelleted by centrifugation at 5,000 rpm. The cells were washed two times with fresh anoxic 100 mM MOPS buffer and then resuspended in the same buffer at pH 7.0. The cell density of the stock culture used to prepare the cell suspensions and to inoculate the experimental cultures was determined using a calibration curve relating cell density to optical absorbance, which was determined by direct counts. Cell suspensions with cell densities of 5 × 108 cells/ml at pH 7.0 were used for all cell suspension experiments, and an initial cell density of 2 × 107 cells/ml was used for growth cultures. Due to material and toxicological constraints, we could provide only enough Pu(IV) to allow for modest cell growth, so it was necessary to use a higher-than-normal initial cell density for the growth experiments. All experimental cultures with Pu species were incubated at 30°C in the dark and constantly shaken at 100 rpm. Cell suspensions containing Pu were incubated using 20-ml sealed serum bottles. The total volume of the cell suspensions was 10.5 ml for all cultures containing Pu.
Enzymatic reduction of Pu(IV) by G. metallireducens and S. oneidensis.
The ability of G. metallireducens and S. oneidensis cell suspensions to enzymatically reduce Pu(IV)(OH)4(am) (with and without EDTA present) and soluble Pu(IV)(EDTA) was assessed by following changes in the Pu(III) concentrations in the cultures over time. For all experiments, an initial concentration of 0.5 mM Pu [for Pu(IV)(OH)4(am), an amount of solid was added such that the aqueous concentration would be 0.5 mM] was added to cell suspensions containing the appropriate electron donor, 10 mM acetate (G. metallireducens) or lactate (S. oneidensis). At each sampling interval, the cultures were sampled anaerobically using sterile syringes with metal needles that had been purged with sterile Ar. The samples were filtered (0.2 μM polytetrafluoroethylene; Millex SLLG013SL syringe filter) directly into vials containing 15 μl concentrated HCl to lower the pH below 1.0. The filtered sample was then mixed with 2.0 M HClO4 in a 1:1 volume ratio, giving a final concentration of 1.0 M HClO4. The concentrations of Pu(III) were determined spectrophotometrically by measuring the maximum absorbance at 600 nm (ɛ = 38 M−1 cm−1) (10). The same procedure was followed to determine Pu(III) concentration in the presence of EDTA. The Pu(III)(EDTA) complex decomposes in strong acid, and the absorbance band at 600 nm is not affected by EDTA in 1.0 M HClO4. In addition to the experimental cultures, three sets of controls were also run concurrently with the experimental cultures. The control cultures consisted of (i) a control with no electron donor, (ii) a control with no cells, and (iii) a control with heat-killed cells. The experimental and control cultures were all conducted in triplicate. The sampling time varied from 15 minutes to 16 hours, depending on the apparent reduction rates and laboratory access limitations.
Growth of G. metallireducens and S. oneidensis with Pu(IV) as the sole terminal electron acceptor.
The ability of G. metallireducens and S. oneidensis to use Pu(IV)(OH)4(am) and Pu(IV)(EDTA) as the sole electron acceptors to support growth was assessed by direct cell counting and protein assay (quantitative cell densities were obtained using a calibration curve based on direct-count data). The growth medium composition was the same as the medium composition described above, with the following exceptions: no NaOH, no Fe(III)-citrate, 10 mM acetate (G. metallireducens) or lactate (S. oneidensis), and 20.92 g/liter MOPS (100 mM). The initial cell density for the growth experiments was 2.0 × 107 cells/ml at pH 7.0. The concentration of Pu in the cultures was fixed at 5.0 mM. All experiments were conducted in triplicate, and controls with no inoculum, no electron donor, and no Pu were also run simultaneously. At each time point, samples were collected as described above. Pu(III) was quantified as described above. Direct cell counting was conducted on an unfiltered sample using a hemocytometer and an optical microscope equipped with a 40× objective. The protein content of the samples was determined using the standard Bradford assay (4).
RESULTS
Reduction of Pu(IV)(OH)4(am) by G. metallireducens and S. oneidensis in cell suspensions.
The data in Fig. 1A and B show the evolution of Pu(III) concentrations over time in cell suspension experiments with Pu(IV)(OH)4(am) provided as the sole terminal electron acceptor. Traces of Pu(III) were observed in the cell suspensions of G. metallireducens incubated with Pu(IV)(OH)4(am) after the first time point. However, at longer incubation times (∼44 h), no Pu(III) could be detected in the cultures. The amounts of Pu(III) observed in the cell suspensions of S. oneidensis (Fig. 1B) were significantly higher than those observed in G. metallireducens. The amount of Pu(IV)(OH)4(am) reduced by the cell suspensions of S. oneidensis was less than 8% of the total Pu present in the cultures.
FIG. 1.
Direct reductions of Pu(OH)4(am) by cell suspensions of G. metallireducens (A) and S. oneidensis (B). Conditions were a cell density of 5 × 108 cells/ml, a pH of 7.0, an acetate concentration of 10 mM (A) or a lactate concentration of 10 mM (B), and a Pu(OH)4(am) concentration of 0.50 mM.
Reduction of Pu(OH)4(am) by G. metallireducens and S. oneidensis in cell suspensions with 0.5 mM EDTA.
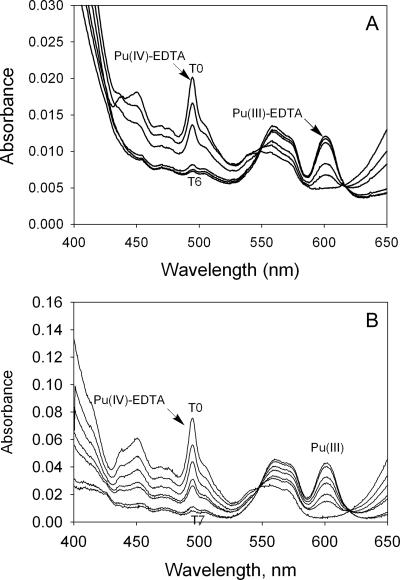
Cell suspensions of both G. metallireducens and S. oneidensis reduced most (approximately 80% and 60%, respectively) of the available Pu(IV)(OH)4(am) in the presence of 0.5 mM EDTA, which was added just prior to the addition of cells to the cultures (Fig. 2A and B). The UV-visible spectra of the acidified filtrates from the S. oneidensis cell suspension experiment are shown in Fig. 3, which shows an increase over time in the absorbance peak for Pu(III) at 600 nm. Aqueous Pu(III) produced in the cell suspensions of S. oneidensis and G. metallireducens in the presence of EDTA remained stable under reducing conditions.
FIG. 2.
Direct reductions of Pu(OH)4(am) by cell suspension of G. metallireducens (A) and S. oneidensis (B) with 0.5 mM EDTA. Conditions were a cell density of 5 × 108 cells/ml, a pH of 7.00, an acetate concentration of 10 mM (A) or a lactate concentration of 10 mM (B), and a Pu(OH)4(am) concentration of 0.50 mM. ▴, live cells with the electron donor; ▵, live cells with no electron donor; ▪, control with no cells; ♦, control with heat-killed cells.
FIG. 3.
Variation of the optical absorbance spectra of acidified solutions containing Pu(OH)4(am) in the presence of a cell suspension of S. oneidensis and 0.5 mM of EDTA. T0, a time point of 15 min; T5, a time point of 72 h. Conditions were a cell density of 5 × 108 cells/ml, a pH of 7.0, a lactate concentration of 10 mM, and a Pu(OH)4(am) concentration of 0.50 mM.
Reduction of the Pu(IV)(EDTA) complex by G. metallireducens and S. oneidensis in cell suspensions.
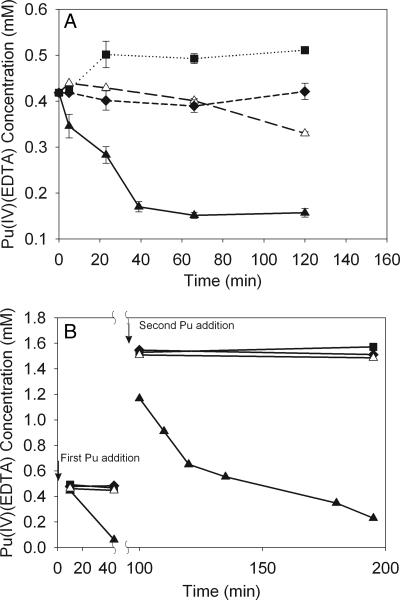
The reduction of Pu(IV)(EDTA) in cell suspensions of both G. metallireducens and S. oneidensis was very rapid (Fig. 4A and B). In cultures with initial concentrations of 0.5 mM Pu(IV)(EDTA), almost all of the Pu(IV)(EDTA) was reduced to Pu(III)(EDTA) in less than 40 min. The production of Pu(III) was not observed in controls with either no cells or heat-killed cells. During the S. oneidensis cell suspension, additional Pu(IV)(EDTA) was added to bring the total concentration to approximately 1.5 mM Pu(IV)(EDTA) in all cultures after all of the first aliquot of Pu(IV) had been reduced. The additional Pu(IV)(EDTA) was also rapidly reduced, and almost all of the Pu(IV)(EDTA) was reduced to Pu(III)(EDTA) within 2 h. The evolution of the Pu(III)/(IV) species concentrations during the cell suspension experiments is shown by the UV-visible spectra in Fig. 5A and B. The data show a gradual decrease of the peak corresponding to Pu(IV)(EDTA) at 495 nm with the concurrent increase in the absorption band corresponding to Pu(III) at 600 nm. The controls with live cells and no electron donor show the production of less Pu(III), whereas the controls with no cells or dead cells show no development of Pu(III).
FIG. 4.
Direct reductions of Pu(IV)(EDTA) by cell suspension of G. metallireducens (A) and S. oneidensis (B). Conditions were a cell density of 5 × 108 cells/ml and a pH of 7.00. (A) Ten millimolar acetate and 0.50 mM Pu(IV)(EDTA) were used. (B) Pu(IV)(EDTA) was added in two steps. The first addition (0.5 mM) was at the point indicated by the arrow, and the second addition (1.0 mM) was at 95 min. The total Pu(IV)(EDTA) added was 1.50 mM. ▴, live cells with the electron donor; ▵, live cells with no electron donor; ▪, control with no cells; ♦, control with heat-killed cells.
FIG. 5.
Variation of the optical absorbance spectra of solutions containing Pu(IV)(EDTA) complex in the presence of cell suspensions of G. metallireducens (A) and S. oneidensis (B). Conditions were a cell density of 5 × 108 cells/ml and a pH of 7.00. (A) Ten millimolar acetate and 0.50 mM Pu(IV)(EDTA); (B) 10 mM lactate and 1.50 mM Pu(IV)(EDTA). The solution was filtered using a 0.2-μm filter to separate the cells from the supernatant and acidified to quantify Pu(IV)(EDTA). (A) T0, a spectrum recorded at 0 min; T6, a spectrum recorded at 120 min. (B) T0, a spectrum recorded at 0 min; T7, a spectrum recorded at 3 h.
Reduction of Pu(IV)(EDTA) and Pu(IV)(OH)4(am) by G. metallireducens and S. oneidensis to support growth.
Significant changes in cell density were not observed, nor was the production of Pu(III) in any growth cultures with Pu(IV)(EDTA) or Pu(IV)(OH)4(am) provided as the sole terminal electron acceptor (data not presented). Growth cultures were monitored for over a week. The concentration of Pu used was sufficient to allow at least a twofold increase in cell density based on the yields obtained for these organisms when Fe(III)-citrate was used.
DISCUSSION
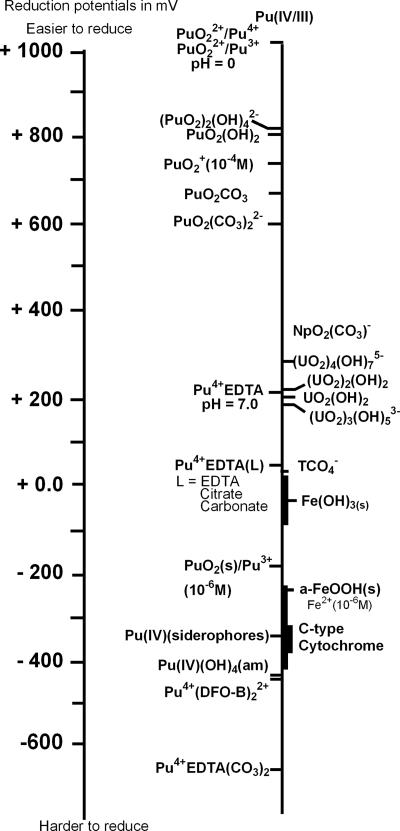
The reduction of Pu(VI) and Pu(V) to Pu(IV) is predicted by analysis of the redox potentials of the species of these oxidation states, which are all within the range accessible to bacterial reduction (Fig. 6). The reduction of Pu(IV) to Pu(III) is more difficult to predict because of the diversity of the species that can form under environmental conditions and the lack of data on the redox accessibility of Pu(IV) species. The reduction potentials of Pu(IV) species are mostly unknown, with the exception of those of a few well-studied ligands, such as EDTA. We estimated the redox potential of the complex Pu(IV)(EDTA) at neutral pH to be +0.217 mV (normal hydrogen electrode) by correcting previously reported values for hydrolysis and estimated the reduction potential of Pu(IV)(OH)4(am) to be −0.41 mV by using existing thermodynamic data. These data are plotted in Fig. 6 along with data for other oxidation states. The data in Fig. 6 show that plutonium can undergo redox changes over a wide range of potentials and that Pu(IV) hydroxide species are situated at the limit of the domain accessible to bacterial reduction. In this study, we show that Pu(IV)(OH)4(am) is accessible to bacterial reduction by G. metallireducens and S. oneidensis. When chelating agents were not present, approximately 8% and 1% of the added Pu(IV)(OH)4(am) in cultures containing S. oneidensis and G. metallireducens, respectively, were converted to soluble Pu(III). Pu(III) was not detected in the control cultures for these experiments. The presence of detectable Pu(III) concentrations in cultures with live bacteria indicates that the biological reduction of Pu(IV)(OH)4(am) occurred in these cultures even though the concentration trends do not show large increases in Pu(III) with time. Concentrations of Pu(III) in the S. oneidensis cultures higher than those observed in the G. metallireducens cultures are consistent with our observations of actinide bio-reduction in general. In all of the actinide systems (Pu, Np, and U) that we studied, S. oneidensis consistently reduced actinides more rapidly and to a greater extent than did G. metallireducens (15).
FIG. 6.
Distribution of the redox potential of plutonium species at neutral pH, along with uranium and iron species included for comparisons. Redox data are from references 8, 10, and 16.
A single previous study also observed what was inferred to be the biological reduction of Pu(IV)(solid) (28). In the previous study, the production of Pu(III) was not observed, but the biological reduction of Pu(IV)(solid) was inferred as a result of an enhanced solubility of Pu with the addition of NTA to cultures containing metal-reducing bacteria. Although only Pu(IV)-NTA complexes were observed, it was speculated that Pu(III) was produced and subsequently oxidized to Pu(IV). Unlike with the results in the previously reported study, we did not observe the reoxidation of Pu(III) in our cultures.
It is unclear whether the lack of higher Pu(III) concentrations observed here in the absence of EDTA was a function of minimal biological reduction or a decrease in reduction as a result of the adsorption of Pu(III) onto cellular and/or Pu(IV)(OH)4(am) surfaces. One possible explanation for the lack of higher Pu(III) concentrations is the limited reduction of Pu(IV)(OH)4(am) in part due to the passivation of the surface by Pu(III). This phenomenon has been observed during the biological reduction of Fe solids (26), where Fe(II) adsorbs onto the surfaces of Fe(III) solids and creates a reduced Fe layer that inhibits further reduction. In the same way, Pu hydrous oxide surfaces rich in binding sites can adsorb unchelated Pu(III) and inhibit further reduction by making Pu(IV) unavailable for reduction. The sorption of free Pu(III) onto G. metallireducens and S. oneidensis cells could also inhibit the reduction of Pu(IV)(OH)4(am) by these bacteria. Additionally, the toxicity of free Pu(III) to these bacteria is unknown and may be significant. The biogeochemistry of uncomplexed Pu(III) is poorly defined, and much additional research is needed to better resolve the behavior of Pu(III) in these systems.
Results from the Pu(IV)(OH)4(am) reduction experiments amended with EDTA indicate that the bacterial reduction of Pu(IV)(OH)4(am) occurred in both cultures with and without EDTA. In the EDTA-amended cultures, most of the Pu(IV)(OH)4(am) added was reduced to Pu(III), which remained as Pu(III) in solution during the experiment. The addition of EDTA, which is a strong complexant for both Pu(IV) and Pu(III) [formation constants (log β110) for Pu(IV)(EDTA) and Pu(III)(EDTA) complexes are 26.44 and 17.06, respectively] (3), can prevent Pu(III) sorption onto cellular or Pu hydrous oxide surfaces by forming a soluble Pu(III)(EDTA) complex. The most likely explanation for these observations is the biological reduction of Pu(IV)(OH)4(am) with the subsequent complexation of Pu(III) by EDTA.
Alternatively, EDTA may have solubilized Pu(IV) as a Pu(IV)(EDTA) complex with the subsequent biological reduction to a Pu(III)(EDTA) complex. The solubilization of Pu(IV) hydrous oxides by chelating ligands is generally not efficient (23, 27). Several reports have investigated the solubilization of Pu(IV) hydrous oxides by chelating ligands, like EDTA, NTA, and natural siderophores (5, 27). The results of these studies indicate that the solubilization can take up to several months to reach equilibrium, and the total concentrations of Pu solubilized from Pu(IV) hydrous oxides are usually in the order of micromoles of Pu solubilized per gram of solid (27). Under the conditions examined in this study, where cell suspensions of G. metallireducens and S. oneidensis were incubated in the presence of EDTA, any amount of solubilized Pu(IV)(EDTA) would rapidly be reduced to Pu(III)(EDTA), which would continually favor more dissolution. However, the lack of measurable concentrations of Pu(IV)(EDTA) in the control cultures during the course of the experiments indicates that the solubilization of Pu(IV) by EDTA was minimal, and therefore, this is not likely to have been a major factor in the reduction of Pu(IV)(OH)4(am).
A third possibility is that EDTA may adsorb to the surfaces of Pu(IV)(OH)4(am) solids and reduce the reduction potential of Pu(IV) at the surface of Pu(IV)(OH)4(am). This could make Pu(IV)(OH)4(am) more easily reducible. In these experiments, it was not possible to determine if EDTA bound to Pu(IV)(OH)4(am) solids or if EDTA binding to Pu(IV)(OH)4(am) solids reduced the reduction potential of Pu(IV) at the surface. However, the reduction potential of Pu(IV)(EDTA) at neutral pH (estimated at +217 mV) is significantly higher than that of Pu(IV) at neutral pH (−0.41 mV), and it is possible that EDTA binding to Pu(IV) atoms present at the surface could make Pu(IV)(OH)4(am) more easily reducible.
The reduction of Pu(IV)(EDTA) to Pu(III)(EDTA) by cell suspensions of G. metallireducens and S. oneidensis is very fast compared to the reduction of Pu(IV)(OH)4(am). The rapid reduction of Pu(IV) in the presence of EDTA shows that ligands can markedly affect the accessibility of Pu(IV) to bacterial reduction. Pu(IV) has a high coordination number (from 9 to 12) and can accommodate up to 12 donor groups from chelating ligands (20, 22). Ternary complexes with ligands such as siderophores tend to have redox potentials too negative for biological reduction and would stabilize Pu(IV). However, the redox potentials of Pu(IV) complexes with most environmentally relevant chelators, such as natural organic acids, humates, polysaccharides, amino carboxylic acids, and synthetic ligands that can potentially be present with plutonium contaminants, are unknown, and it is not known if chelators would facilitate the bio-reduction of Pu(IV) hydroxides.
Growth was not observed for either G. metallireducens or S. oneidensis when Pu(IV)(EDTA) and Pu(IV)(OH)4(am) were provided as sole terminal electron acceptors. It is possible that under conditions other than those examined here, these bacteria may be able to utilize Pu(IV)(EDTA) or Pu(IV)(OH)4(am) for growth. However, it is more likely that these organisms are not able to support growth by utilizing Pu(IV)(EDTA) or Pu(IV)(OH)4(am), which is not an uncommon observation for other metal systems and bacteria (19).
Implications of the bio-reduction of Pu hydrous oxides for the fate and transport of Pu.
We have demonstrated that Pu(IV) can be reduced to Pu(III) by direct enzymatic reduction, but much work remains to be done to fully understand the consequences of Pu hydrous oxide reduction for the fate and transport of Pu in the environment. The in situ approach consisting of the biological reduction and the subsequent precipitation of actinides that has been recommended as a remedial strategy for U-contaminated subsurface environments (1) may be more difficult to implement for Pu-contaminated sites. Our results indicate that strongly anaerobic conditions may increase the solubility of Pu. In many areas, Pu contamination is present primarily as insoluble Pu(IV) hydrous oxides or associated with colloids (21). If these Pu(IV) solids are subjected to anaerobic conditions under which metal-reducing bacteria dominate, the concentration of soluble Pu could increase, especially if chelating ligands are present. Natural chelators are abundant in many environments (13), especially organically rich reducing environments. In addition to natural ligands, synthetic ligands, such as EDTA and NTA, are common cocontaminants at Pu-contaminated sites (25). Both natural and synthetic ligands may play an important role in mobilizing plutonium under reducing conditions.
In an anaerobic environment (metal or sulfate reducing), the primary substrates will likely be either Fe(III)-oxyhydroxides, Mn(IV)-oxides, or sulfate with the production of the reduced species (Fe2+, Mn2+, and S−2). These reduced species are very soluble and may react with oxidized Pu species to create more reduced Pu species. A recent study has demonstrated that it is possible to reduce PuO2(am) to Pu(III) with both Fe(II) and hydroquinone (a redox-active compound that metal-reducing bacteria can reduce) (22). It is therefore possible that the indirect reduction of Pu species by other reduced species may have a big impact on Pu solubility and mobility. As with the direct enzymatic reduction of Pu(IV), the presence of complexing ligands would have the potential to significantly enhance the mobility of Pu in these environments. A recent investigation of the stability of U(IV) species under anaerobic conditions in the presence of a naturally occurring chelator (desferrioxamine B) showed that the complex U(IV)(desferrioxamine B) remained soluble (14). This emphasizes the importance of bacterial metabolites and cocontaminants for the fate and transport of actinide species in the environment.
Acknowledgments
This work was supported by the Environmental Bioremediation Sciences Program within the U.S. Department of Energy Office of Science.
Footnotes
Published ahead of print on 20 July 2007.
REFERENCES
- 1.Anderson, R. T., H. A. Vrionis, I. Ortiz-Bernad, C. T. Resch, P. E. Long, R. Dayvault, K. Karp, S. Marutzky, D. R. Metzler, A. Peacock, D. C. White, M. Lowe, and D. R. Lovley. 2003. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 69:5884-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boukhalfa, H., S. D. Reilly, and M. P. Neu. 2007. Complexation of Pu(IV) with the natural siderophore desferrioxamine B and the redox properties of Pu(IV)(siderophore) complexes. Inorg. Chem. 46:1018-1026. [DOI] [PubMed] [Google Scholar]
- 3.Boukhalfa, H., S. D. Reilly, W. H. Smith, and M. P. Neu. 2004. EDTA and mixed-ligand complexes of tetravalent and trivalent plutonium. Inorg. Chem. 43:5816-5823. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Brainard, J. R., B. A. Strietelmeier, P. H. Smith, P. J. Langston-Unkefer, M. E. Barr, and R. R. Ryan. 1992. Actinide binding and solubilization by microbial siderophores. Radiochim. Acta 58-59:357-363. [Google Scholar]
- 6.Choppin, G. 2003. Actinide speciation in the environment. Radiochim. Acta 91:645-649. [Google Scholar]
- 7.Reference deleted.
- 8.Choppin, G. R., A. H. Bond, and P. M. Hromadka. 1997. Redox speciation of plutonium. J. Radioanal. Nuc. Chem. 219:203-210. [Google Scholar]
- 9.Clark, D. L., D. R. Janecky, and L. J. Lane. 2006. Science-based cleanup of Rocky Flats. Physics Today 59:34-40. [Google Scholar]
- 10.Cleveland, J. M. 1979. The chemistry of plutonium. The American Nuclear Society, La Grange Park, IL.
- 11.Cleveland, J. M., and T. F. Rees. 1981. Characterization of plutonium in Maxey Flats radioactive trench leachates. Science 212:1506-1509. [DOI] [PubMed] [Google Scholar]
- 12.Cowan, C. E., E. A. Jenne, D. E. Robertson, D. M. Nelson, and K. H. Abel. 1985. Transuranic chemical species in ground water: final report. PNL-5263. Pacific Northwest Laboratory, Richland, WA.
- 13.Fox, T. R., and N. B. Comerford. 1990. Low-molecular-weight organic acids in selected forest soils of the southeastern USA. Soil Sci. Soc. Am. J. 54:1139-1144. [Google Scholar]
- 14.Frazier, S. W., R. Kretzschmar, and S. M. Kraemer. 2005. Bacterial siderophores promote dissolution of UO2 under reducing conditions. Environ. Sci. Technol. 39:5709-5715. [DOI] [PubMed] [Google Scholar]
- 15.Icopini, G. A., H. Boukhalfa, and M. P. Neu. 2007. Biological reduction of Np(V) and Np(V) citrate by metal-reducing bacteria. Environ. Sci. Technol. 41:2764-2769. [DOI] [PubMed] [Google Scholar]
- 16.Icopini, G. A., H. Boukhalfa, and M. P. Neu. 2006. Environmental reduction of Tc, U, Np, and Pu by bacteria and the stability of reduction products, p. 20-25. In R. Alvarez, N. D. Bryan, and I. May (ed.), Recent advances in actinide science. Royal Society of Chemistry, Cambridge, United Kingdom.
- 17.Knopp, R., V. Neck, and J. I. Kim. 1999. Solubility, hydrolysis and colloid formation of plutonium(IV). Radiochim. Acta 86:101-108. [Google Scholar]
- 18.Lovley, D. R. 2003. Cleaning up with genomics: applying molecular biology to bioremediation. Nat. Rev. Microbiol. 1:36-44. [DOI] [PubMed] [Google Scholar]
- 19.Lovley, D. R., E. E. Roden, E. J. P. Phillips, and J. C. Woodward. 1993. Enzymatic iron and uranium reduction by sulfate-reducing bacteria. Mar. Geol. 113:41-53. [Google Scholar]
- 20.Matonic, J. H., M. P. Neu, A. E. Enriquez, R. T. Paine, and B. L. Scott. 2002. Synthesis and crystal structure of a ten-coordinate plutonium(IV) ion complexed by 2-[(diphenylphosphino)methyl]pyridine N,P-dioxide: [Pu(NO3)3{2-[(C6H5)2P(O)CH2]C5H4NO}2][Pu(NO3)6]0.5. J. Chem. Soc. Dalton Trans. 11:2328-2332. [Google Scholar]
- 21.McCarthy, J. F., and J. M. Zachara. 1989. Subsurface transport of contaminants. Environ. Sci. Technol. 23:496-502. [Google Scholar]
- 22.Neu, M., J. Matonic, C. Ruggiero, and B. Scott. 2000. Structural characterization of a plutonium(IV) siderophore complex: single-crystal structure of Pu-desferrioxamine E. Angew. Chem. Int. Ed. Engl. 39:1442-1444. [DOI] [PubMed] [Google Scholar]
- 23.Rai, D., Y. A. Gorby, J. K. Fredrickson, D. A. Moore, and M. Yui. 2002. Reductive dissolution of PuO2(am): the effect of Fe(II) and hydroquinone. J. Solut. Chem. 31:433-453. [Google Scholar]
- 24.Reilly, D. S., and M. P. Neu. 2006. Pu(VI) hydrolysis: further evidence for a dimeric plutonyl hydroxide and contrast with U(VI). Inorg. Chem. 45:1839-1846. [DOI] [PubMed] [Google Scholar]
- 25.Riley, R. G., and J. M. Zachara. 1992. Chemical contaminants on DOE lands and selection of contaminant mixtures for subsurface science research. Report no. DOE/ER-0547T. Department of Energy, Office of Energy Research, Subsurface Science Program, Washington, DC.
- 26.Royer, R. A., B. A. Dempsey, B. H. Jeon, and W. D. Burgos. 2004. Inhibition of biological reductive dissolution of hematite by ferrous iron. Environ. Sci. Technol. 38:187-193. [DOI] [PubMed] [Google Scholar]
- 27.Ruggiero, C. E., J. H. Matonic, S. D. Reilly, and M. P. Neu. 2002. Dissolution of plutonium(IV) hydroxide by desferrioxamine siderophores and simple organic chelators. Inorg. Chem. 41:3593-3595. [DOI] [PubMed] [Google Scholar]
- 28.Rusin, P. A., L. Quintana, J. R. Brainard, B. A. Strietelmeier, C. D. Tait, S. A. Ekberg, P. D. Palmer, T. W. Newton, and D. L. Clark. 1994. Solubilization of plutonium hydrous oxide by iron-reducing bacteria. Environ. Sci. Technol. 28:1686-1690. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki, Y., S. D. Kelly, K. M. Kemner, and J. F. Banfield. 2002. Radionuclide contamination: nanometre-size products of uranium bioreduction. Nature 419:134. [DOI] [PubMed] [Google Scholar]