Abstract
In this study, we present a novel method to isolate and enrich low concentrations of Campylobacter pathogens. This method, Acanthamoeba-Campylobacter coculture (ACC), is based on the intracellular survival and multiplication of Campylobacter species in the free-living protozoan Acanthamoeba polyphaga. Four of the Campylobacter species relevant to humans and livestock, Campylobacter jejuni, C. coli, C. lari, and C. hyointestinalis, were effectively enriched by the coculture method, with growth rates comparable to those observed in other Campylobacter enrichment media. Studying six strains of C. jejuni isolated from different sources, we found that all of the strains could be enriched from an inoculum of fewer than 10 bacteria. The sensitivity of the ACC method was not negatively affected by the use of Campylobacter-selective antibiotics in the culture medium, but these were effective in suppressing the growth of seven different bacterial species added at a concentration of 104 CFU/ml of each species as deliberate contamination. The ACC method has advantages over other enrichment methods as it is not dependent on a microaerobic milieu and does not require the use of blood or other oxygen-quenching agents. Our study found the ACC method to be a promising tool for the enrichment of Campylobacter species, particularly from water samples with low bacterial concentrations.
The genus Campylobacter contains several species that cause disease in humans and livestock. With regard to humans, Campylobacter jejuni and C. coli are the two most important species. However, C. lari, C. hyointestinalis, and C. fetus are also recognized as important human pathogens, although less commonly (1, 7, 18, 19). Typically, patients with acute campylobacteriosis develop gastroenteritis and excrete high numbers of Campylobacter bacteria in their feces. Detecting an infection at this stage is comparably straightforward on Campylobacter-selective agar plates (4, 15, 23), although competing bacterial flora may interfere. However, isolation of Campylobacter from food sources, environmental water samples, or clinical samples in the late phase of infection is more complicated (4, 12, 13, 15), as these samples often contain only low concentrations of bacteria, a larger proportion of which might be injured and aged cells. In addition, such samples often have problems with competing microbial flora that overgrow the less competitive Campylobacter bacteria.
Detection of Campylobacter from nonclinical samples therefore often involves an enrichment step in a Campylobacter-specific broth before plating on blood agar, in order to increase the yield of bacteria (4, 6). Some of the recommended and most widely used enrichment broths are Preston broth and Campylobacter enrichment broth (CEB [Bolton formula]) (14). These broths are reported to have high performance in terms of the ability to enrich old and injured Campylobacter cells, as well as in suppressing the growth of competitor organisms (3). They contain Campylobacter-selective antibiotics, as well as blood and other oxygen-quenching agents.
We have recently shown that C. jejuni is able to invade and survive and multiply within the protozoan Acanthamoeba polyphaga (2). This observation was confirmed with the related species A. castellanii (24). Here, we build on this knowledge and present Acanthamoeba-Campylobacter coculture (ACC) as a novel enrichment method for Campylobacter spp. We show that the ACC method is a powerful tool to detect C. jejuni and other thermotolerant Campylobacter cells at concentrations far below the detection limit of blood agar culture, and we compare its sensitivity and selectivity with those of recommended broths for Campylobacter enrichment. In contrast to conventional culture methods for Campylobacter spp., the ACC method does not require the use of blood or a microaerobic gas environment.
MATERIALS AND METHODS
ACC method.
The ACC method rests on previous observations (2) that C. jejuni strains of diverse sources are capable of infecting A. polyphaga cells in vitro at temperatures ranging from +4 to +37°C. At low temperatures, the bacteria enter and remain viable within the amoeba. An increase in temperature to 37°C results in massive replication of the intracellular campylobacters, with subsequent lysis of the infected amoebae.
Our experimental setup is illustrated in Fig. 1. A. polyphaga (strain Linc Ap-1) was stored as cysts in peptone-yeast extract-glucose (PYG) medium at +4°C. Trophozoites were maintained aerobically at 27°C in PYG medium, at the bottom of 25-cm2 culture flasks. The PYG medium was prepared according to Rowbotham (22), with later modifications of Greub and Raoult (8). A 5-liter stock of PYG medium consists of 100 g of proteose peptone (Becton Dickinson), 10 g of yeast extract (Scharlau), 4.9 g of MgSO4 · 7H2O, 5 g of sodium citrate · 2H2O, 0.1 g of Fe(NH4)2(SO4)2 · 6H2O, 1.7 g of KH2PO4, 1.97 g of Na2HPO4 · 7H2O, 45 g of glucose, and 0.295 g of CaCl2 (Sigma Aldrich). The ingredients were dissolved in distilled water, filtered through a Munktell no. 5 filter paper (Munktell, Grycksbo, Sweden), and autoclaved (15 min, 121°C).
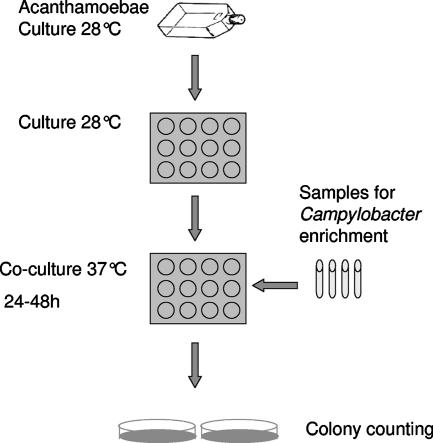
FIG. 1.
ACC method. Trophozoites of A. polyphaga maintained in PYG medium are seeded onto 12-well plates and allowed to form a confluent layer. Before coculture, the medium is changed and samples to be tested for the growth of Campylobacter spp. are added. The coculture plate is then incubated for 24 to 48 h to allow enrichment of Campylobacter bacteria. The presence of Campylobacter cells is confirmed by plating onto blood agar.
Three days prior to each experiment, the A. polyphaga trophozoites were seeded in 12-well culture plates and grown aerobically at 27°C to reach a confluent layer. Before seeding, the trophozoites were detached from the culture flask by incubation in 4 ml of fresh medium at −20°C, allowing the liquid to freeze. Thereafter, the flask was tapped a few times against the palm.
Just before each experiment, the medium in all wells was gently replaced with fresh PYG medium with a pipette without disturbing the amoeba layer at the bottom of each well. For coculturing experiments, trophozoites were inoculated with Campylobacter and incubated aerobically at 37°C for optimal growth of Campylobacter cells. Growth of Campylobacter was confirmed by microscopic observation of a small sample from each well, as well as by plating 100 μl on blood agar. The plates were observed for colony and bacterial morphology after incubation in a microaerobic environment for 24 h at 42°C (37°C for C. hyointestinalis). The capacity of the ACC method to enrich Campylobacter spp. was assessed by using six C. jejuni strains of different origins and one strain each of the species C. coli, C. lari, and C. hyointestinalis. The collection numbers and origins of the strains used here and throughout this study were as follows: C. jejuni CCUG 11284, a reference strain originally isolated from a human patient; C. jejuni F4382 and F6555, human isolates from Swedish patients with gastrointestinal campylobacteriosis; C. jejuni 1153 and 6137, Swedish chicken isolates; C. jejuni 02A364, obtained from a wild mallard (Anas platyrhynchos) sampled in Sweden; C. coli LMG 6440, a pig isolate; C. lari LMG 8846, an avian isolate (Larus argentatus); and C. hyointestinalis CCUG 20822, a human isolate.
Growth kinetics of Campylobacter spp. in the ACC method.
We investigated the growth kinetics of three different Campylobacter species in the ACC method over the course of 96 h. Strains of C. jejuni (CCUG 11284), C. coli, and C. lari were cultured on conventional blood agar (Columbia agar II containing 8% [vol/vol] whole horse blood) at 42°C in a microaerobic gas environment, with the CampyGen gas-generating system (CN0025A; Oxoid, Ltd., Basingstoke, United Kingdom) and the BBL GasPak system (BD, Franklin Lakes, NJ). Bacterial cells were harvested, suspended in PYG medium, and diluted to a final concentration of approximately 103 CFU/ml, as determined by direct plating on blood agar plates and counting of colonies formed after 24 h under microaerobic conditions.
Confluent cultures of A. polyphaga grown at the bottom of a 12-well plate in 1.9 ml of PYG medium were inoculated with 100 μl of the bacterial stock solutions in triplicate. The ACC plate was then incubated aerobically at 37°C, and samples of 100 μl were taken every sixth hour until 72 h, with an additional and final sample at 96 h. A control plate containing PYG medium but no A. polyphaga was also inoculated with 100 μl of the bacterial stock solutions in triplicate. The plate was treated identically to the test plates, except that samples were taken at 24-h intervals because no growth was expected. The samples from each time point were diluted in 10-fold dilution series, and 100 μl from each dilution step was spread onto blood agar plates. The plates were incubated microaerobically for 24 h, and the colonies formed were counted.
Sensitivity and selectivity of the ACC method compared with those of conventional enrichment broths.
To investigate the potential of the ACC method as a detection tool for Campylobacter spp., we compared the sensitivity and selectivity of the ACC method with those of the commonly used Preston broth (nutrient broth no. 2 [CM0067; Oxoid, Ltd., Basingstoke, United Kingdom]) and CEB (Bolton formulation [LAB135; LABM, Bury, United Kingdom]), both supplemented with 5% (vol/vol) lysed horse blood. The broths were used without the addition of selective antibiotics, except in the selectivity tests. Sensitivity was measured as the lower detection limits of the methods, and selectivity was measured as the different methods' abilities to detect Campylobacter cells in samples with competing microbial flora.
In both experiments, ACC was incubated aerobically at 37°C whereas the broths were incubated according to the U.S. FDA bacteriological analytical manual (14), in a microaerobic atmosphere at 42°C. For the sensitivity analyses, we used six strains of C. jejuni of different origins (described above), whereas in the selectivity experiments only the reference strain, C. jejuni CCUG 11284, was used.
Strains from seven different bacterial species were used as competing flora in the selectivity experiments. These included Streptococcus pyogenes (CCUG 23117), Burkholderia cepacia (CCUG 13226), Moraxella catarrhalis (CCUG 34455), Pasteurella multocida (CCUG 224), Pseudomonas aeruginosa (CCUG 17619), Escherichia coli (ATCC 25922), and Bacillus subtilis (CCUG 163B). The strains were chosen to represent a diverse spectrum of bacteria from those abundant in feces, water, and soil to those present as infectious agents or commensals in humans and livestock. All of the bacterial strains were stored frozen at −80°C, and before an experiment they were thawed and grown on blood agar plates, harvested, and suspended in PYG medium. The Campylobacter strains were cultured under microaerobic conditions as described above; all other bacteria were grown on blood agar under aerobic conditions at 37°C for 24 h. Each experiment determining sensitivity and selectivity was conducted twice for each Campylobacter strain.
Sensitivity experiment setup.
The general setup of the sensitivity experiment was as follows. Forty-eight-hour-old cultures of each Campylobacter strain were harvested, suspended individually in fresh PYG medium, and further diluted 10-fold to obtain series where at least one dilution step in each series contained no bacteria. From each dilution step, 100 μl was inoculated in triplicate to each of the three enrichment methods, the ACC method, Preston broth, and CEB. At the same time, 100 μl from each dilution step was also plated directly onto blood agar plates for determination of the bacterial concentration. For all of the methods, the bacteria were inoculated into 1.5 ml of medium in 12-well culture plates. In the ACC, the PYG medium was replaced with 1.5 ml of fresh medium just before inoculation. The ACC plates were incubated at 37°C aerobically, whereas the plates with CEB and Preston broth were incubated at 42°C in a microaerobic environment.
Initially and after 24, 48, 72, and 96 h, a 100-μl sample of each culture was plated on blood agar and incubated in a microaerobic environment at 42°C for 24 h. The culturability of each strain by the different methods was assessed by counting the colonies on the plates. Because of the large number of plates, growth was classified as weak (1 to 10 CFU), intermediate (10 to 100 CFU), or rich (>100 CFU).
Selectivity experiment setup.
To test the three methods for selectivity, we conducted an experiment similar to the one described above but with the addition of Campylobacter-selective antibiotics and competing flora. In these experiments, modified Bolton broth selective supplement (SR0208; Oxoid, Ltd., Basingstoke, United Kingdom) containing cefoperazone (10.0 mg), trimethoprim (10.0 mg), vancomycin (10.0 mg), and amphotericin B (5.0 mg) was used for the CEB and modified Preston Campylobacter selective supplement (SR0204E, Oxoid Ltd., Basingstoke, United Kingdom) containing polymyxin B (2,500 IU), rifampin (5.0 mg), trimethoprim (5.0 mg), and amphotericin B (5.0 mg) was used for Preston broth. The modified Bolton selective supplement was chosen for the ACC method after an initial evaluation of this supplement and two other antibiotic mixtures, the Preston Campylobacter selective supplement and a mixture of cefoperazone (16.0 mg) and amphotericin B (5.0 mg) (X112 LAB M). The latter two were abandoned because of severe overgrowth of the contaminating bacteria.
This experiment was performed in two steps and only with the reference strain C. jejuni CCUG 11284. In the first step, dilutions of strain CCUG 11284 were inoculated in triplicate by the three different culture methods. Culturability was quantitatively assessed with the same settings as described for the sensitivity experiments to determine whether the antibiotics affected the detection capacities of the methods.
In the second step, dilutions of CCUG 11284 were added in triplicate to the three culture methods in the presence of a mixture of competing bacteria. This mixture contained the bacteria S. pyogenes, B. cepacia, M. catarrhalis, P. multocida, P. aeruginosa, E. coli, and B. subtilis at 104 CFU/ml. The culture methods were incubated and sampled for blood agar plating as described above. The Campylobacter colonies on the plates were counted, as well as the number of colonies from contaminants. Campylobacter colonies were verified by microscopic observation.
To determine the abilities of the methods to suppress contaminating bacteria in a natural setting, each method was inoculated with water samples from seven different environmental sources. This experiment had the same setup as described above, except that 1.5 ml of medium containing antibiotics was used and 500 μl of each water sample was added to the wells in five replicates. The bacterial contents of the water samples were also determined by culture on blood agar at 37°C aerobically for 24 h.
Statistics.
Minimum generation times for C. jejuni, C. coli, and C. lari growth in the ACC method were calculated from the maximum slopes over a 24-h period. These statistical analyses were based on data from two similar experiments, each of which was performed in triplicate for every strain tested. Duncan tests were performed to determine if there were differences between the minimum generation times of the Campylobacter species tested.
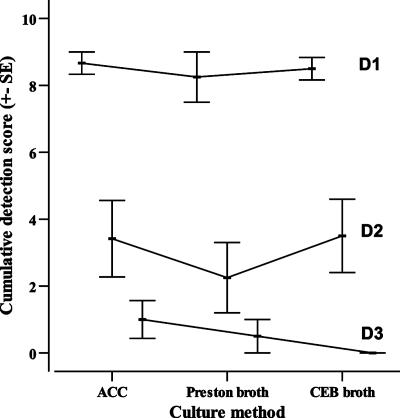
The sensitivities of the three culture methods, ACC, Preston broth, and CEB, were compared by assessing their enrichment capacities for three different inocula representing consecutive 10-fold serial dilutions of a bacterial stock. These dilutions were designated D1, D2, and D3. At each dilution step (D1 to D3), the enrichment capacities of the methods were determined from the outcome of the plate counts after the enrichment step. Colony counts from the plates were judged as weak, intermediate, or rich growth and correspondingly ranked 1, 2, or 3 for nonparametric statistic analyses. The performance was calculated for each dilution step (D1 to D3) as the sum of the growth ranks of three replicates for each of the Campylobacter strains tested. That is, for each dilution step a method can have a score ranging from 0, in cases where there was no growth in any of the triplicates at that dilution step, to 9, in cases where there was rich growth in all of the triplicates. The average ranks from all six strains for a given dilution step were added for each method, giving each method a total rank for each of the three dilution steps of the inoculum (see Fig. 3).
FIG. 3.
Cumulative detection score from the sensitivity experiment. The sensitivity of the ACC method was assessed with six different C. jejuni strains and compared to those of commercially available enrichment methods, i.e., Preston broth and CEB. Each method was given a score depending on its ability to enrich a defined inoculum of three different concentration ranges. The plot shows the cumulative detection score for each method and the concentration range (D1 to D3). There were no significant differences between the detection scores from each concentration range for the different enrichment methods (D1, H = 0.380, n = 36, P = 0.83 [Kruskal-Wallis test]; D2, H = 1.088, n = 36, P = 0.58; D3, H = 3.638, n = 36, P = 0.16). SE, standard error.
We used nonparametric Kruskal-Wallis analysis of variance to test if there were statistically significant differences between the detection capacities of the different methods (Statistica, version 7.1; Statsoft). This test compares the outcomes of the three methods by summing the performance of the six strains tested, combining the results from all triplicates for every strain at each dilution step for two repeated experiments. The software SPSS (version 14.0 for Windows) was used to plot the performance of the different methods.
RESULTS
Enrichment of Campylobacter spp. by the ACC method.
We assessed the potential of the ACC method as a tool for the enrichment of Campylobacter spp. by using six strains of C. jejuni and one strain each of C. coli, C. lari, and C. hyointestinalis. All of the strains tested were successfully enriched by the method. Although inocula with bacterial contents of fewer than 10 CFU (as determined by direct plating) were used, the strains were enriched to a density of >103 CFU/ml in 24 h. These results show that the method is effective at enriching Campylobacter spp. of high relevance for humans and livestock.
Growth kinetics of Campylobacter species by the ACC method.
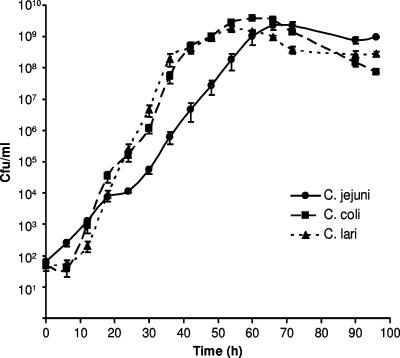
The growth kinetics of three Campylobacter species were determined for the ACC method (Fig. 2). All of the strains exhibited exponential growth to a density of approximately 109 CFU/ml, and maximum concentrations were reached within 48 to 60 h. All of the strains reached concentrations of >104 CFU/ml by 24 h. The minimum generation times under exponential growth were 2.03 h for C. jejuni (standard deviation [SD] = 0.23 h), 1.45 h for C. coli (SD = 0.06 h), and 1.25 h for C. lari (SD = 0.12 h). Growth rates differed significantly between the species tested (analysis of variance, followed by Duncan's test: C. jejuni versus C. coli, P < 0.001; C. jejuni versus C. lari, P < 0.0001; C. coli versus C. lari, P < 0.05).
FIG. 2.
Growth kinetics of C. jejuni, C. coli, and C. lari by the ACC method. The graph shows mean values from two experiments performed in triplicate for each strain ± the standard errors of the means.
Once peak densities were reached, concentrations started to decline for all of the strains, probably reflecting an impaired survival of extracellular bacteria in the PYG medium. None of the strains grew in the control plate containing PYG medium only.
Sensitivity of the ACC method compared with that of conventional enrichment broths.
The sensitivity of the ACC method in detecting Campylobacter was compared to those of Preston broth and CEB for six strains of C. jejuni. The bacteria were diluted in 10-fold dilution series before inoculation of the culture methods, and the comparison was based on three consecutive dilution steps, D1 to D3. For all of the strains, the D1 dilution step gave intermediate (11 to 100 CFU/100 μl) or weak (1 to 10 CFU/100 μl) growth, as determined by direct plating on blood agar. This gave a maximum inoculum of all of the strains of 100 CFU for the D1 dilution step and hence 10 CFU for D2 and 1 CFU for D3.
The general sensitivity of the methods was not affected by prolonged coculture/enrichment times, meaning that the results at 24 h were similar to the results at 48 h, 72 h, and 96 h (data not shown). Therefore, we restricted statistical analyses to the results obtained at 24 h of growth. Overall, the ACC method was as sensitive as the two conventional enrichment broths in detecting C. jejuni and no statistically significant differences were seen at any of the dilution steps (D1, n = 36, P = 0.83 [Kruskal-Wallis test]; D2, n = 36, P = 0.58; D3, n = 36, P = 0.16; Fig. 3). All three methods propagated an inoculum of fewer than 10 bacteria per well to a density of >103 CFU/ml in 24 h. The ACC method performed slightly better than CEB and Preston broth with small inocula (1 CFU and 11 to 100 CFU, respectively), but these differences were not statistically significant (Fig. 3). The method that was most sensitive differed slightly between C. jejuni strains, but none of the methods consistently performed best.
Comparison of the selectivities of enrichment methods.
The selectivity of the ACC method was compared to those of the Preston broth and CEB, i.e., their abilities to support the growth of C. jejuni while suppressing the growth of competitor organisms. The experiment was performed only with the C. jejuni CCUG 11284 strain in the presence of Campylobacter selective antibiotics, with or without the addition of a contaminating bacterial flora.
We could not detect any inhibitory effect on the sensitivity of the three culture methods due to the presence of the antibiotics, and there were no apparent differences in sensitivity between the methods, although the sample size did not allow statistical analysis (Table 1).
TABLE 1.
C. jejuni detection capacities, in the presence or absence of competing bacterial flora, of the ACC, CEB, and Preston broth methods supplemented with antibiotics
| Expt and dilution | No. of wells that detected C. jejunia
|
|||||
|---|---|---|---|---|---|---|
| Without competing flora
|
With competing flora
|
|||||
| ACC | CEB | Preston broth | ACC | CEB | Preston broth | |
| 1 | ||||||
| 1 | 3 | 3 | 3 | 3 | 3 | 0b |
| 2 | 3 | 2 | 3 | 2 | 2 | 0b |
| 3 | 0 | 0 | 0 | 0 | 0 | 0a |
| 2 | ||||||
| 1 | 3 | 3 | 3 | 3 | 3 | 0b |
| 2 | 2 | 2 | 2 | 1 | 2c | 0b |
| 3 | 0 | 0 | 0 | 0 | 0 | 0b |
As determined by growth on blood agar plates after plating of 100 μl in triplicate.
All plates were overgrown with competing flora.
One plate was overgrown with competing flora.
In the presence of the contaminating microbial flora, the ACC method and CEB propagated an inoculum of <10 bacteria per well to a density of >103 CFU/ml in 24 h, with no apparent differences between the two (Table 1), and with the growth of only a few colonies of contaminating bacteria (Table 2). In Preston broth, however, it was not possible to estimate the efficiency of specific propagation of C. jejuni because of severe overgrowth of contaminating bacteria.
TABLE 2.
Growth of microflora competing with Campylobacter by the ACC, CEB, or Preston broth method
| Expt and dilution | No. of colonies on blood agar platesa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ACC
|
CEB
|
Preston broth
|
|||||||
| Replicate 1 | Replicate 2 | Replicate 3 | Replicate 1 | Replicate 2 | Replicate 3 | Replicate 1 | Replicate 2 | Replicate 3 | |
| 1 | |||||||||
| 1 | 12 | 3 | 6 | Ob | O | O | |||
| 2 | 1 | 4 | O | O | O | ||||
| 3 | 2 | O | O | O | |||||
| 2 | |||||||||
| 1 | 1 | 2 | 2 | 1 | 1 | O | O | O | |
| 2 | 1 | 1 | O | 1 | 2 | O | O | O | |
| 3 | 2 | 1 | 3 | O | O | O | |||
A volume of 100 μl was plated per plate.
O denotes total overgrowth by competing flora, precluding assessment.
The abilities of the methods to suppress the growth of contaminating bacteria were further determined with environmental water samples. Natural bacterial flora was detected in six out of seven samples by aerobic culture on blood agar at 37°C, but only a few colonies were detected after an enrichment step with the ACC or the other methods.
DISCUSSION
We have presented a novel tool for the enrichment of Campylobacter spp, the ACC method. It is a quick, simple, and sensitive method. Samples presumed to contain Campylobacter are seeded into a small volume of PYG medium containing A. polyphaga trophozoites and incubated aerobically at 37°C for 24 h. During this time, the Campylobacter cells enter and proliferate at a rapid rate inside the amoebae. Transferring a small volume of the cocultured sample to a normal blood agar plate, combined with additional microaerobic incubation for 24 h, yields a large number of Campylobacter colonies for detection and further characterization (Fig. 1).
We successfully amplified six diverse strains of C. jejuni, as well as single strains of C. coli, C. lari, and C. hyointestinalis, by the ACC method. The species tested here are those most important from a human health perspective, covering more than 99% of the reported cases of human infection (14). The four Campylobacter species grew surprisingly fast in the amoebae, with generation times within the range reported for C. jejuni in other culture media, i.e., varying from 1.5 h to 2.2 h, depending on the growth medium and strain used (17, 20). These results suggest that the growth conditions for the campylobacters inside the amoebas are close to optimal, despite a lower temperature and a lack of artificially provided microaerobic conditions.
We compared the sensitivity and selectivity of the ACC method with those of two conventional enrichment broths commonly used in bacteriological laboratories, CEB and Preston broth. First, we tested differences in detection capacity with six C. jejuni strains isolated from wild birds, patients, and poultry with no antibiotic supplement. The ACC method and the two broths successfully enriched inocula of approximately 10 bacterial cells to densities of >103 CFU/ml in 24 h, and the ACC method was as sensitive as Preston broth and CEB in this respect. However, under most circumstances, an unknown environmental or patient sample will contain a rich microbial flora from which the fastidious campylobacters will likely face competition. To evaluate the usefulness of the ACC method with such diverse samples, we tested its performance by adding antibiotics and competing flora. We started to test whether supplementation of Campylobacter-selective antibiotics affected the viability of the amoeba trophozoites and found that neither amoebae nor C. jejuni bacteria were negatively affected by the antibiotics.
We tested the detection performance of the ACC method in the presence of contaminating bacteria, because an important prerequisite for a selective culture method is the ability to suppress the growth of other organisms without impairing the detection of the intended one. The antibiotic mixture we chose for the study was effective by the ACC method, because we were able to isolate C. jejuni in a background of seven different competing microorganisms at a concentration of 104 CFU/ml for each organism. The sensitivity in terms of the size of the inoculum needed for successful growth was not affected by the presence of antibiotics or contaminating microorganisms. When the sensitivity and selectivity in the background of contaminating organisms were compared to those of CEB and Preston broth, the ACC method performed equally well or better. The test with environmental water samples further proved that the method was efficient in suppressing the growth of contaminating organisms present in such samples.
In conclusion, the ACC method is a very promising culture method for Campylobacter. We have shown that it performs excellently with small inocula of bacteria, even with a background of competing flora, and that common Campylobacter-specific antibiotics do not interfere substantially with its detection capacity. Acanthamoeba cells are easily maintained in culture, and to establish a monolayer for coculture requires only 1 to 2 days after subculture, depending on the size of the inoculum. Thus, it is easy to have amoebae “up and running” in anticipation of samples. Furthermore, the incubation step does not need a microaerobic gas environment, which means that the method is affordable for all laboratories. In this article, we have provided the basic methodology and rationale for the ACC method. Further testing of environmental and clinical samples will determine its value in practical use.
During the past 2 decades, a growing body of evidence has documented the intracellular survival of bacteria in free-living protozoans (9, 11, 25), including a number of pathogens affecting human health such as Legionella spp. (22), Mycobacterium spp. (26), and Helicobacter spp. (29). Two previous studies (2, 24) have shown that Campylobacter cells readily infect amoebae and can proliferate intracellularly in protozoan hosts given that certain environmental conditions are met. We observed that C. jejuni survived in A. polyphaga for more that 60 days at 10°C. In this report, we showed that three species of Campylobacter all grew with close-to-optimal kinetics within A. polyphaga at 37°C. This finding adds to the hypothesis that protozoans may be important as intermediate hosts for Campylobacter spp., possibly explaining the puzzling distribution of the bacterium in many environments, such as surface and ground waters (10, 16, 21), where it should not be able to grow. The importance of a water reservoir for Campylobacter is further indicated by the high prevalence rates of C. jejuni and C. lari in aquatic wild birds (5, 27, 28).
As far as we know, our study is the first where the methodology of protozoan-bacterium coculture was used as a detection method for Campylobacter spp. However, similar methods have been applied to isolate amoeba-resistant intracellular microorganisms by using free-living amoebae (9). We believe that amoeba-bacterium coculture methods could be set up for other fastidious bacteria and are a promising arena for future research.
Acknowledgments
This work was supported financially by the Royal Swedish Academy of Agriculture and Forestry (KSLA-SF450); the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS 2004-856); the Health Research Council of Southeast Sweden (FORSS), Sparbankstiftelsen Kronan; and the Carl Trygger Foundation.
The amoeba strain used in this study was provided by Bernard La Scola, Université de la Méditerranée, Marseille, France.
Footnotes
Published ahead of print on 14 September 2007.
REFERENCES
- 1.Altekruse, S. F., N. J. Stern, P. I. Fields, and D. L. Swerdlow. 1999. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axelsson-Olsson, D., J. Waldenstrom, T. Broman, B. Olsen, and M. Holmberg. 2005. Protozoan Acanthamoeba polyphaga as a potential reservoir for Campylobacter jejuni. Appl. Environ. Microbiol. 71:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baylis, C. L., S. MacPhee, K. W. Martin, T. J. Humphrey, and R. P. Betts. 2000. Comparison of three enrichment media for the isolation of Campylobacter spp. from foods. J. Appl. Microbiol. 89:884-891. [DOI] [PubMed] [Google Scholar]
- 4.Bolton, F. J., and L. Robertson. 1982. A selective medium for isolating Campylobacter jejuni/coli. J. Clin. Pathol. 35:462-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broman, T., H. Palmgren, S. Bergstrom, M. Sellin, J. Waldenstrom, M. L. Danielsson-Tham, and B. Olsen. 2002. Campylobacter jejuni in black-headed gulls (Larus ridibundus): prevalence, genotypes, and influence on C. jejuni epidemiology. J. Clin. Microbiol. 40:4594-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corry, J. E., D. E. Post, P. Colin, and M. J. Laisney. 1995. Culture media for the isolation of campylobacters. Int. J. Food Microbiol. 26:43-76. [DOI] [PubMed] [Google Scholar]
- 7.Gebhart, C. J., P. Edmonds, G. E. Ward, H. J. Kurtz, and D. J. Brenner. 1985. “Campylobacter hyointestinalis” sp. nov.: a new species of Campylobacter found in the intestines of pigs and other animals. J. Clin. Microbiol. 21:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greub, G., and D. Raoult. 2002. Crescent bodies of Parachlamydia acanthamoeba and its life cycle within Acanthamoeba polyphaga: an electron micrograph study. Appl. Environ. Microbiol. 68:3076-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greub, G., and D. Raoult. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hänninen, M. L., H. Haajanen, T. Pummi, K. Wermundsen, M. L. Katila, H. Sarkkinen, I. Miettinen, and H. Rautelin. 2003. Detection and typing of Campylobacter jejuni and Campylobacter coli and analysis of indicator organisms in three waterborne outbreaks in Finland. Appl. Environ. Microbiol. 69:1391-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harf, C. 1994. Free-living amoeba: interactions with environmental pathogenic bacteria. Endocytobiosis Cell Res. 10:167-183. [Google Scholar]
- 12.Humphrey, T. J. 1989. An appraisal of the efficacy of pre-enrichment for the isolation of Campylobacter jejuni from water and food. J. Appl. Bacteriol. 66:119-126. [DOI] [PubMed] [Google Scholar]
- 13.Humphrey, T. J. 1986. Techniques for the optimum recovery of cold injured Campylobacter jejuni from milk or water. J. Appl. Bacteriol. 61:125-132. [DOI] [PubMed] [Google Scholar]
- 14.Hunt, J. M., A. C., and T. T. 1998. Campylobacter. In J. M. Hunt, C. Abeyta, and T. Tran (ed.), FDA bacteriological analytical manual, 8th ed. U.S. Food and Drug Administration, Washington, DC. http://www.cfsan.fda.gov/∼ebam/bam-7.html.
- 15.Hutchinson, D. N., and F. J. Bolton. 1983. Is enrichment culture necessary for the isolation of Campylobacter jejuni from faeces? J. Clin. Pathol. 36:1350-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, K., M. Betaieb, and D. R. Telford. 1990. Thermophilic campylobacters in surface waters around Lancaster, UK: negative correlation with Campylobacter infections in the community. J. Appl. Bacteriol. 69:758-764. [DOI] [PubMed] [Google Scholar]
- 17.Khanna, M. R., S. P. Bhavsar, and B. P. Kapadnis. 2006. Effect of temperature on growth and chemotactic behaviour of Campylobacter jejuni. Lett. Appl. Microbiol. 43:84-90. [DOI] [PubMed] [Google Scholar]
- 18.Lawson, A. J., J. M. Logan, L. O'Neill, G. M. Desai, and J. Stanley. 1999. Large-scale survey of Campylobacter species in human gastroenteritis by PCR and PCR-enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:3860-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore, J. E., D. Corcoran, J. S. Dooley, S. Fanning, B. Lucey, M. Matsuda, D. A. McDowell, F. Megraud, B. C. Millar, R. O'Mahony, L. O'Riordan, M. O'Rourke, J. R. Rao, P. J. Rooney, A. Sails, and P. Whyte. 2005. Campylobacter. Vet. Res. 36:351-382. [DOI] [PubMed] [Google Scholar]
- 20.Rollins, D. M., J. C. Coolbaugh, R. I. Walker, and E. Weiss. 1983. Biphasic culture system for rapid Campylobacter cultivation. Appl. Environ. Microbiol. 45:284-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosef, O., G. Rettedal, and L. Lageide. 2001. Thermophilic campylobacters in surface water: a potential risk of campylobacteriosis. Int. J. Environ. Health Res. 11:321-327. [DOI] [PubMed] [Google Scholar]
- 22.Rowbotham, T. J. 1983. Isolation of Legionella pneumophila from clinical specimens via amoebae, and the interaction of those and other isolates with amoebae. J. Clin. Pathol. 36:978-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skirrow, M. B. 1977. Campylobacter enteritis: a “new” disease. BMJ 2:9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snelling, W. J., J. P. McKenna, D. M. Lecky, and J. S. Dooley. 2005. Survival of Campylobacter jejuni in waterborne protozoa. Appl. Environ. Microbiol. 71:5560-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snelling, W. J., J. E. Moore, J. P. McKenna, D. M. Lecky, and J. S. Dooley. 2006. Bacterial-protozoa interactions; an update on the role these phenomena play towards human illness. Microbes Infect. 8:578-587. [DOI] [PubMed] [Google Scholar]
- 26.Steinert, M., K. Birkness, E. White, B. Fields, and F. Quinn. 1998. Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl. Environ. Microbiol. 64:2256-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldenström, J., T. Broman, I. Carlsson, D. Hasselquist, R. P. Achterberg, J. A. Wagenaar, and B. Olsen. 2002. Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in different ecological guilds and taxa of migrating birds. Appl. Environ. Microbiol. 68:5911-5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whelan, C. D., P. Monaghan, R. W. Girdwood, and C. R. Fricker. 1988. The significance of wild birds (Larus sp.) in the epidemiology of Campylobacter infections in humans. Epidemiol. Infect. 101:259-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winiecka-Krusnell, J., K. Wreiber, A. von Euler, L. Engstrand, and E. Linder. 2002. Free-living amoebae promote growth and survival of Helicobacter pylori. Scand. J. Infect. Dis. 34:253-256. [DOI] [PubMed] [Google Scholar]